Molecular geometry is dictated by VSEPR theory. A central atom’s bond angles can be predicted number of substituents and lone pairs directly attached.
VSEPR theory:
Valence Shell Electron Pair Repulsion theory is based on the idea that groups of atoms and electrons will repel each other as much as possible. This repulsion results in particular optimal bond angles, which in turn result in molecular geometries.
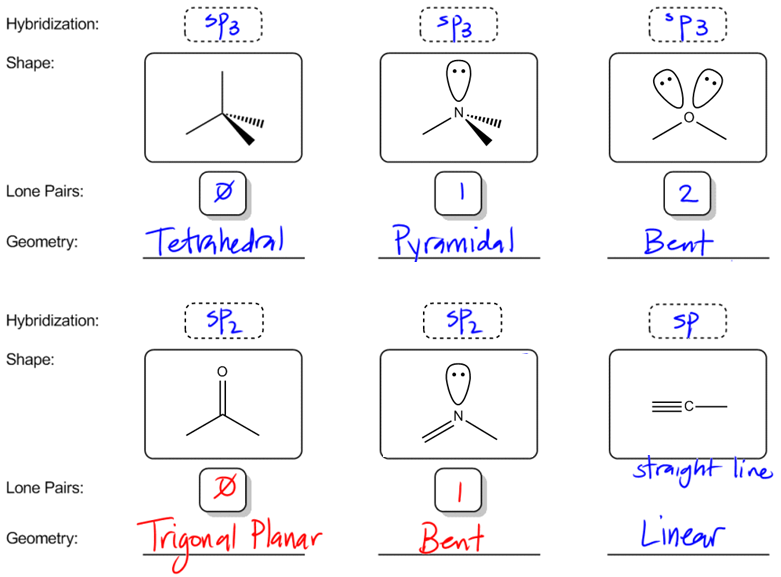
Using bonding preferences and hybridization of a central atom, we can accurately predict the molecular geometry (fancy way of saying molecular shape). Hybridization can be discerned by the number of groups (atoms and lone pairs) attached to an atom.
Hybridization:
The hybridization of an atom can help us quickly determine the shape of a molecule. Hybridization of a central atom is determined by how many bonds and/or lone pairs that atom has. Collectively, lone pairs and bonds are called bond sites. Empty orbitals do not count as bond sites!
4 bond sites = sp3 hybridized
3 bond sites = sp2 hybridized
2 bond sites = sp hybridized
Bonding preferences
Atoms of different groups (columns of the periodic table) will actually have different numbers of bonds and lone pairs in their neutral states. Here’s a pretty helpful chart to show what I mean. Each atom is drawn in its neutral state—that is, with no charge.

Bonding preferences
Bonding preferences are extremely helpful when determining the hybridization of atoms because your professor might not draw lone pairs or hydrogens. Generally, bondline structure omits hydrogens attached to carbon atoms and lone pairs attached to heteroatoms (any atom that isn’t a carbon or hydrogen). If no charge is indicated, it’s assumed that the atom meets its bonding preference through “implied” hydrogens or lone pairs.
Molecular Geometry
Molecular Geometry is determined by the number of groups attached to a central atom. Think about it this way: what’s the farthest two bond sites can be separated if they’re attached to the same central atom? 180º apart. That’s what we would refer to as linear geometry. Remember that atoms and lone pairs will repel each other as far away as possible. They just want equal elbow room, basically.
Of course, things get a bit more complicated when there are more bond sites to consider. Let’s take a look at neutral boron. Boron likes to have three atoms attached and no lone pairs, so what’s the maximum angle between the atoms attached?

Boron bond angles
Using X to represent any atom, let’s think about this. If boron has three groups attached, the absolute maximum angle at which the three groups can be distributed is 120º. Hang on a second… that means that the molecule will actually be flat! This is what we refer to as trigonal planar geometry, and it occurs with three bond sites and zero lone pairs.
Instead of going through each iteration, let’s try to understand the repulsive effects of lone pairs and atoms attached to a central atom.

C, N, O hybridization and geometries
Notice that the nitrogen has two different categories of sp3. A neutral nitrogen generally has three bonds and one lone pair (4 groups total) and a cationic nitrogen generally has four bonds and no lone pairs. The presence or absence of that lone pair makes a difference on the geometry of the molecule. Let’s use that chart to find the hybridizations and geometries of the central atoms in the following molecules:

Geometries blank
Try these out on your own before looking below for the answers. Hint: remember that lone pairs can make geometry a little bit different.

Geometries answers
To do this accurately, we need to do three things:
1) Find how many bonds and/or lone pairs the central atom has
2) Use that information to determine the hybridization of the central atom
3) Use the number of bonds and lone pairs to determine the geometry
We talked earlier about how lone pairs can affect geometry. Notice that carbon (top left) and nitrogen (top middle) each have for bonding sites but different geometry. Basically, you can imagine the bonds as legs or sides of the shape and the lone pair as a little cap.
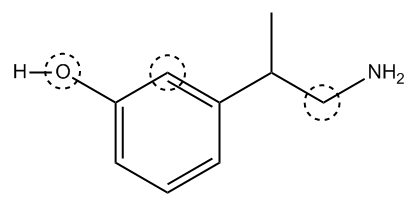
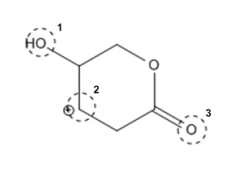
Let’s try finding the hybridization and geometry of three atoms circled on this next molecule:

Hybridizations and geometries of three atoms blank
Try this one on your own before looking at the answer below. All lone pairs and hydrogens attached to carbon atoms have been implied. Heads up! Notice that there is a carbon with a positive charge. What does that mean? How many bonds does it have? How many lone pairs?

Hybridizations and geometries of three atoms answered
Both oxygen molecules have two bonds and two lone pairs, but they have different hybridization. Why? Because they have a different number of atoms attached; the oxygen on the left has single bonds to a carbon and to a hydrogen while the oxygen on the right has both bonds to a single carbon.
The carbon is a bit more complicated, though. Check it out. It’s got two bonds to carbon that are drawn explicitly and it’s got a bond to an implied hydrogen attached. It has zero lone pairs. That means it has a total of three bond sites; remember that empty orbitals like that in the carbocation do not count as bond sites.
That wasn’t so bad, right? So, let’s try another—potentially weirder—example. Let’s take a look at the different hybridizations and geometries of NH3, BF3, and AlCl3. Remember that boron is in group 3 (to the left of carbon) and aluminum is right underneath boron.

Ammonia, boron triflouoride, and aluminum trichloride geometry
NH3 is sp3, BF3 is sp2, and AlCl3 is sp2. Even though they all have three bonds, the geometries are different. The nitrogen’s geometry is trigonal pyramidal while the geometry of both boron and aluminum is trigonal planar. Again, that’s because of that lone pair on the nitrogen. Imagine we somehow added a lone pair to the boron. What would happen? The lone pair would repulse (push away) the fluorines and achieve a trigonal pyramidal structure. If we added another fluorine instead of the lone pair, a tetrahedral geometry would develop.
Let’s try to figure out the molecular geometries of a few more. How about the central atoms of NH4+, CH4, H2O, CO2, and H2CO? Try drawing the Lewis structures of each on your own.

A few more examples
Both the sp3 nitrogen and sp3 carbon have tetrahedral geometries since they have four bonds and no lone pairs; the oxygen in water has two bonds and two lone pairs, so it’s sp3 and bent; the carbon in formaldehyde has three atoms attached and no lone pairs, so it’s sp2 and trigonal planar; and the carbon in carbon dioxide has only two atoms attached and no lone pairs, so it has a linear geometry.