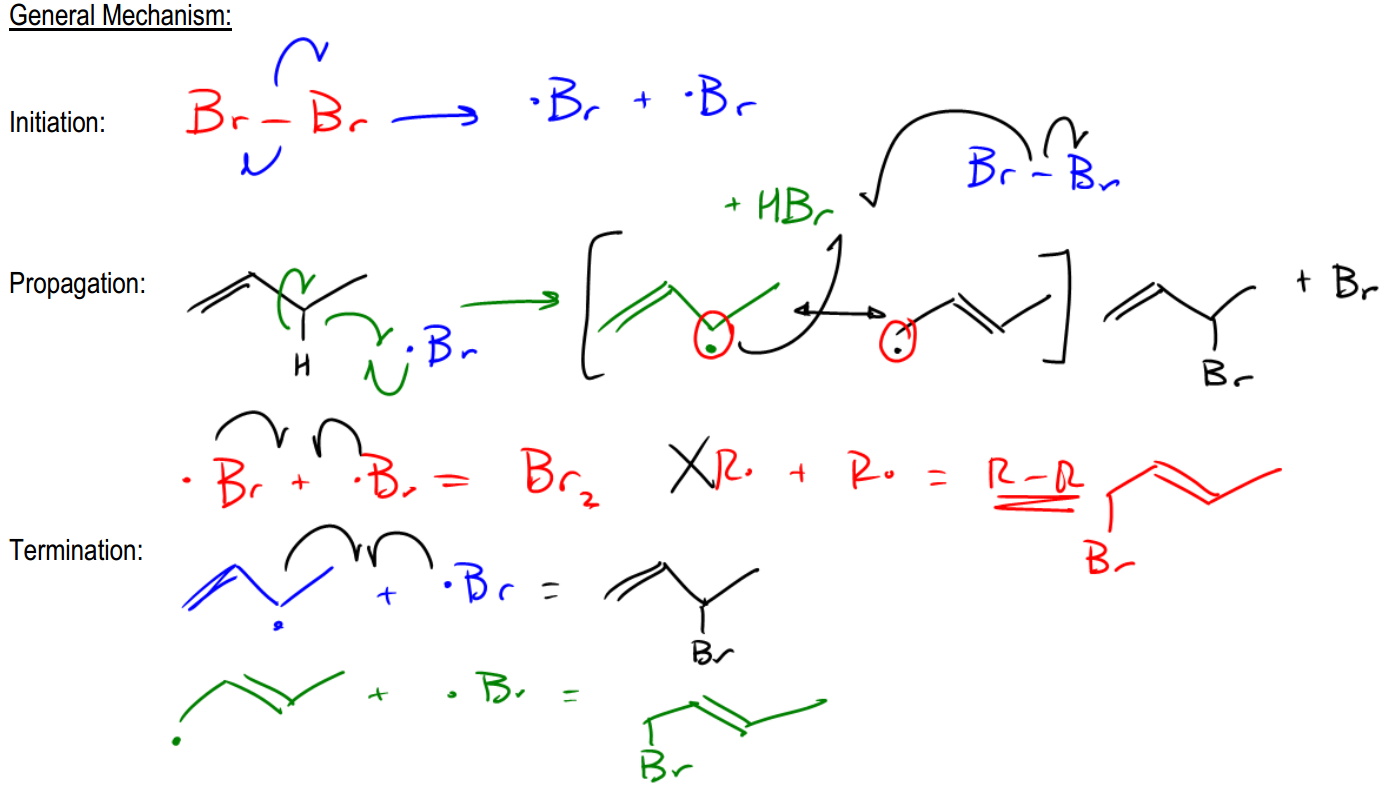
In the study of radical halogenation mechanisms, it is essential to understand the stability of radicals, particularly when considering allylic positions. Radicals are attracted to sites with higher stability, which follows the trend that tertiary radicals are more stable than primary ones. However, allylic radicals, which are adjacent to double bonds, exhibit even greater stability than primary, secondary, or tertiary radicals. This stability is crucial when discussing allylic halogenation, where the presence of allylic and benzylic positions significantly influences the reaction outcome.
During allylic halogenation, the mechanism begins with the initiation step, typically involving a diatomic halogen such as bromine (Br2). The initiation generates halogen radicals, which are then involved in the propagation step. In this phase, the halogen radical abstracts a hydrogen atom from the allylic position, leading to the formation of a more stable radical. The resonance of this radical plays a pivotal role, as it allows the radical to delocalize, creating multiple resonance structures. This delocalization means that the radical can react at more than one position, leading to a mixture of products.
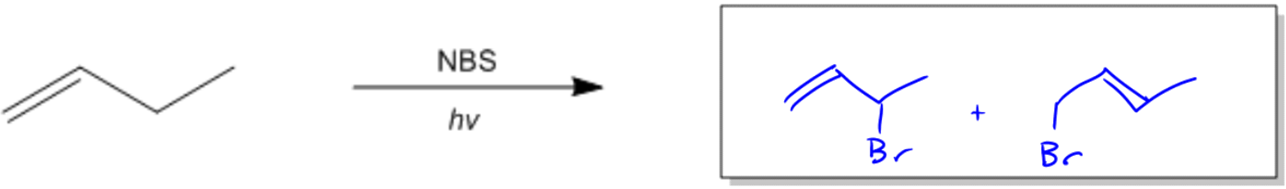
For example, when bromine reacts with an alkene, the halogen radical can attack the allylic carbon, resulting in the formation of a radical that can resonate between two positions. This resonance allows for the possibility of generating two distinct products, as the radical can stabilize itself by shifting between the two carbons adjacent to the double bond. The resulting products from this reaction will include both possible halogenated structures, reflecting the statistical nature of resonance rather than a strict equilibrium.
The termination step of the reaction can involve various combinations of radicals, leading to the formation of stable products. It is important to note that in allylic halogenation, the presence of resonance structures is what differentiates it from other halogenation processes, such as those involving only tertiary or secondary positions, which typically yield a single product. Understanding the resonance and the potential for multiple products is key to mastering allylic halogenation mechanisms.
In summary, allylic halogenation is characterized by the stability of allylic radicals, the significance of resonance in determining reaction pathways, and the generation of a mixture of products due to the ability of the radical to delocalize. Recognizing these factors is essential for predicting the outcomes of reactions involving allylic halogenation.