Electronegativity is a fundamental concept in chemistry that describes the ability of an atom to attract shared electrons in a chemical bond. When two atoms form a bond by sharing their valence electrons, the extent of this sharing can vary significantly, leading to bonds of different strengths and characteristics. The unequal sharing of electrons creates a dipole moment, which is a measure of the separation of positive and negative charges in a bond. The dipole moment (\( \mu \)) can be calculated using the formula:
\[ \mu = q \cdot r \]
where \( q \) is the charge difference between the two atoms and \( r \) is the distance between them. The charge difference is closely related to the difference in electronegativity between the two atoms involved in the bond.
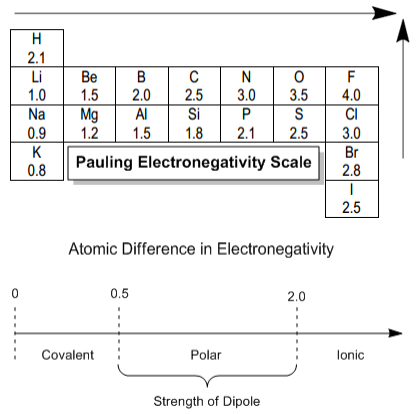
The Pauling Electronegativity Scale is commonly used to quantify electronegativity values, with fluorine being the most electronegative element at 4.0. The scale helps categorize bonds based on the difference in electronegativity. For instance, if the difference is less than 0.5, the bond is considered covalent, indicating significant sharing of electrons. An example of this is a bond between two fluorine atoms, both having an electronegativity of 4.0, resulting in equal sharing of electrons.
When the difference in electronegativity is between 0.5 and 2.0, the bond is classified as polar covalent. In this case, one atom attracts the shared electrons more strongly than the other, leading to a dipole moment. For example, in a bond between carbon (electronegativity 2.5) and fluorine (electronegativity 4.0), fluorine pulls the electrons closer, resulting in a partial negative charge (\( \delta^- \)) on fluorine and a partial positive charge (\( \delta^+ \)) on carbon.
On the other hand, if the electronegativity difference exceeds 2.0, the bond is considered ionic, where there is minimal sharing of electrons. A classic example is sodium chloride (NaCl), where sodium (electronegativity 0.9) and chlorine (electronegativity 3.0) form an ionic bond, resulting in a complete transfer of electrons from sodium to chlorine, creating Na+ and Cl- ions.
Understanding the spectrum of bond types—covalent, polar covalent, and ionic—allows for better predictions of molecular behavior. General rules can simplify the identification of bond types: bonds between carbon and hydrogen are typically covalent, bonds between identical atoms are always covalent, adjacent atoms on the periodic table tend to form polar bonds, and lone pairs also contribute to polarity.
Finally, the concept of net dipole moments arises when there are asymmetrical dipole moments in a molecule. If the individual dipole moments do not cancel each other out, the molecule exhibits a net dipole, which can influence its physical and chemical properties. Understanding these principles is crucial for analyzing molecular interactions and behaviors in organic chemistry.