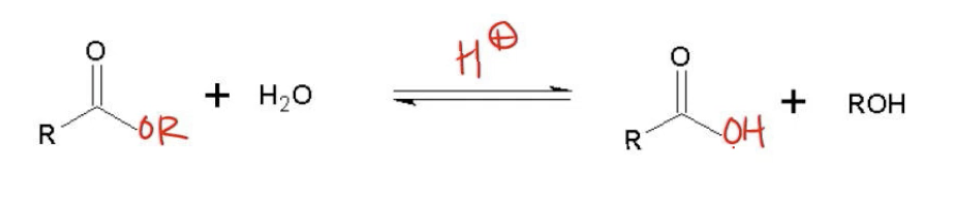
Acid catalyzed ester hydrolysis is a chemical reaction that effectively reverses Fischer esterification, allowing for the conversion of an ester back into a carboxylic acid. In this process, an ester reacts with water in the presence of an acid catalyst, leading to the formation of a carboxylic acid. The general reaction can be represented as follows:
\[ \text{RCOOR'} + \text{H}_2\text{O} \xrightarrow{\text{H}^+} \text{RCOOH} + \text{R'OH} \]
In this equation, RCOOR' represents the ester, H2O is water, RCOOH is the resulting carboxylic acid, and R'OH is the alcohol produced. This reaction aligns with the third rule of nucleophilic acyl substitution (NAS), which states that any carboxylic acid derivative, when combined with water and an acid or base, can be converted into a carboxylic acid.
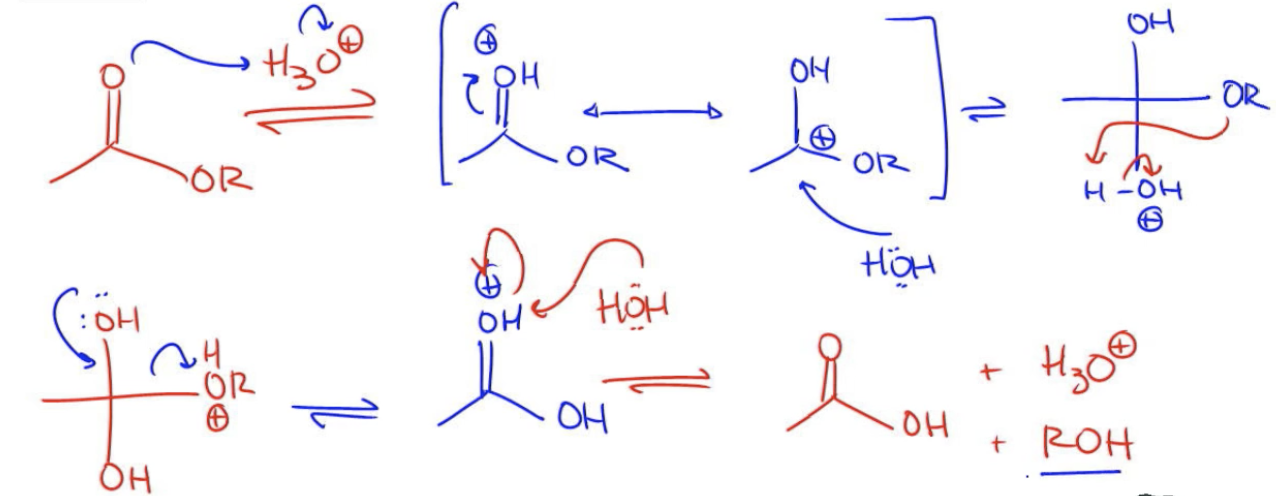
To understand the mechanism of acid catalyzed ester hydrolysis, one can visualize it as the reverse of Fischer esterification. In Fischer esterification, a carboxylic acid and an alcohol react to form an ester and water. Therefore, to illustrate the hydrolysis mechanism, one would essentially trace the steps of Fischer esterification in reverse. This involves identifying the nucleophiles and electrophiles and drawing the reaction arrows in the opposite direction.
By comprehending this mechanism, students can appreciate the dynamic nature of ester chemistry and the interconversion between esters and carboxylic acids, which is fundamental in organic synthesis and various biochemical processes.