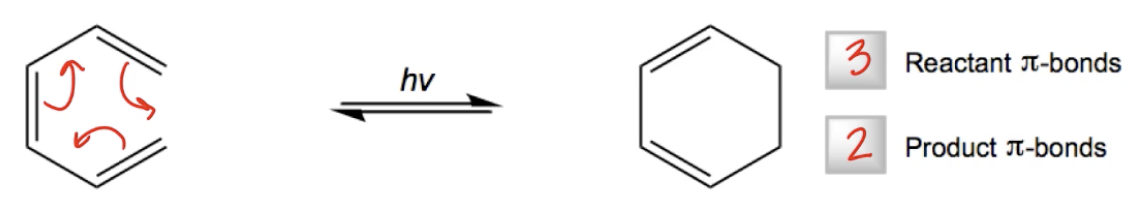
Photochemical electrocyclic reactions are a specific type of intramolecular pericyclic reaction characterized by the destruction of one pi bond through a light-activated cyclic mechanism. In these reactions, a molecule reacts with itself under the influence of light, resulting in the formation of a new ring structure while converting one pi bond into a sigma bond. This process is applicable to all conjugated polyenes, highlighting its broad relevance in organic chemistry.
The mechanism of a photochemical electrocyclic reaction is similar to that of a thermal electrocyclic reaction, with the primary difference being the involvement of light energy. This energy excites ground state electrons, promoting them to a higher energy level, typically from a bonding molecular orbital (HOMO) to an antibonding molecular orbital (LUMO). The change in the identity of these orbitals is crucial for determining the stereochemistry of the product.
In a typical diene, the molecular orbital diagram consists of filled orbitals with four electrons occupying the first two bonding orbitals (Ψ1 and Ψ2). The highest occupied molecular orbital (HOMO) is usually Ψ2, while the lowest unoccupied molecular orbital (LUMO) is Ψ3. Upon exposure to light, one electron is excited to a higher energy state, altering the HOMO and LUMO designations. After excitation, the new HOMO becomes Ψ3 and the new LUMO becomes Ψ4. However, for the purpose of the electrocyclic reaction, only the HOMO is relevant since the reaction is intramolecular.
Understanding the changes in the molecular orbitals due to light activation is essential for predicting the stereochemistry of the resulting product. The next steps involve applying this knowledge to draw the stereochemistry of an electrocyclic reaction influenced by light, emphasizing the importance of frontier molecular orbital theory in these transformations.