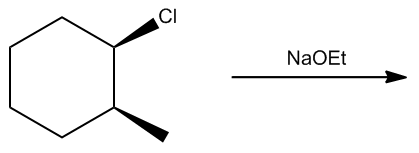
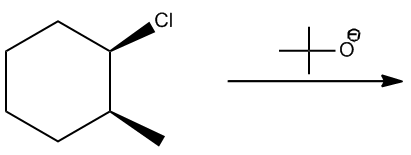
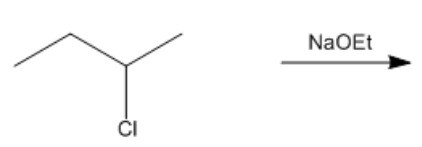
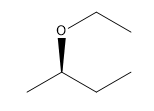
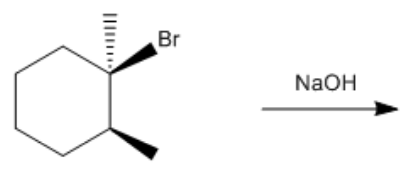
In the study of alkyl halides and their reactions with nucleophiles, understanding the mechanism is crucial. When considering a nucleophile like sodium ethoxide (NaOEt), it is important to recognize that it dissociates to form a negatively charged ethoxide ion (OEt-). This negatively charged nucleophile directs the reaction towards the left side of the mechanism flowchart.
Next, we assess whether NaOEt is a bulky base. It is not classified as one of the bulky bases, which helps narrow down the possible mechanisms. The type of alkyl halide is also significant; in this case, the carbon is attached to two other carbons, indicating it is a secondary alkyl halide. The final consideration is whether the base is a better nucleophile or a better base. Since NaOEt is a strong base, it favors an E2 elimination mechanism.
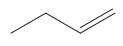
In an E2 reaction, the first step involves identifying the beta protons available for elimination. Here, there are two beta carbons, each with hydrogen atoms that can participate in the reaction. However, only certain beta protons can react according to the anti-coplanar rule, which states that the leaving group and the hydrogen being eliminated must be on opposite sides of the molecule. In this scenario, two beta protons can react, leading to the formation of two potential products.
To illustrate the mechanism, we can use arrows to show the movement of electrons. For instance, if we eliminate a hydrogen from one beta carbon, we form a double bond while expelling the leaving group (Cl-). This results in a product with a double bond and a specific stereochemistry, which is important for accurately representing the molecule. The stereochemistry is determined by the arrangement of groups around the carbon atoms involved in the double bond.
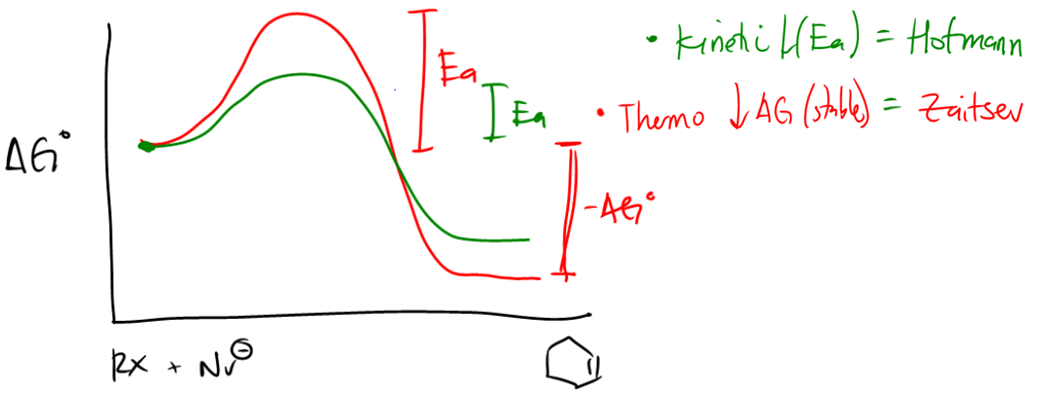
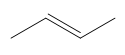
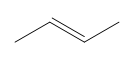
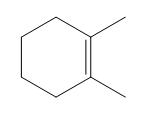
According to Zaitsev's rule, the more substituted alkene product is favored, as it is thermodynamically more stable. In this case, the product with the trisubstituted double bond is more stable than the disubstituted one. Therefore, the major product of the reaction will be the one that is more substituted, while the less substituted product will be the minor product. It is essential to note that both products can be formed, but in different amounts, with the major product being the one that predominates.
In summary, when analyzing reactions involving alkyl halides and nucleophiles, it is vital to determine the mechanism, identify the beta protons, and apply Zaitsev's rule to predict the major and minor products accurately. This systematic approach not only clarifies the reaction pathway but also enhances understanding of the stability of the resulting alkenes.