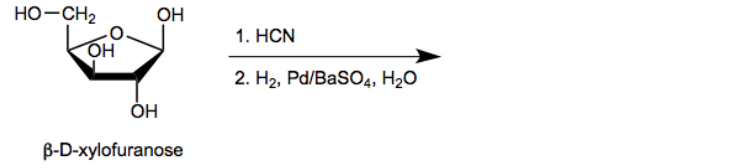
The Kiliani-Fischer Synthesis is a method used to extend monosaccharide chains, transforming simpler sugars into more complex forms. This process leverages the versatility of saccharides, which contain functional groups such as alcohols and carbonyls. A key aspect of this synthesis is the nucleophilic addition reaction involving aldehydes, where hydrogen cyanide (HCN) reacts with an aldehyde to form a cyanohydrin. This reaction is reminiscent of the mechanisms studied in carbonyl chemistry, where the cyanide ion (CN-) attacks the carbonyl carbon, resulting in a new functional group.
To extend the carbon chain, the cyano group can be hydrolyzed and reduced to form a new aldehyde. The reduction of the cyano group typically involves a selective reducing agent that converts the cyano group (C≡N) to an imine (C=N) without fully reducing it to an amine (C-NH2). This imine can then undergo hydrolysis, converting the nitrogen to an oxygen and yielding a new aldehyde. The overall reaction can be summarized as follows:
1. Formation of cyanohydrin:
RCHO + HCN → RCH(OH)C≡N
2. Reduction of cyano group:
RCH(OH)C≡N → RCH(OH)C=N (using a selective reducing agent)
3. Hydrolysis of imine:
RCH(OH)C=N + H2O → RCH(OH)C=O + NH3
This cycle can be repeated multiple times, allowing for the transformation of a pentose sugar into a hexose or even longer chains. However, a notable limitation of the Kiliani-Fischer Synthesis is the generation of a mixture of stereoisomers at each chiral center introduced during the process. As the synthesis progresses, the chirality at certain carbons becomes ambiguous, leading to a mixture of configurations. This results in a blend of diastereomers, which may exhibit different physical properties, complicating the purification and characterization of the final product.
In summary, the Kiliani-Fischer Synthesis is a powerful technique for elongating monosaccharide chains, utilizing established reactions from carbonyl chemistry while introducing challenges related to stereochemistry and product purity.