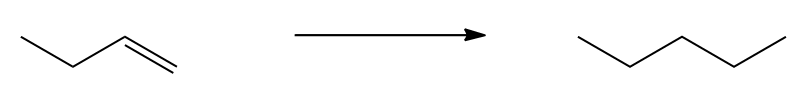
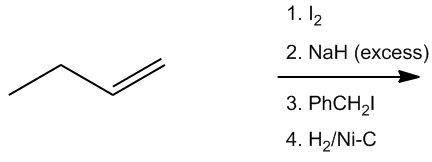In this synthesis example, we start with a terminal alkyne, which already contains a triple bond, simplifying the process. The first step involves deprotonating the terminal alkyne using a strong base, such as sodium amide (NH2), to generate an alkynide ion. This alkynide ion is a negatively charged nucleophile that can participate in further reactions.
Next, the alkynide ion reacts with a primary leaving group in a nucleophilic substitution reaction. The flowchart method is useful here to determine the mechanism. Since we have a strong nucleophile and a primary leaving group, the reaction follows the SN2 mechanism. In this mechanism, the alkynide performs a backside attack on the alkyl halide, resulting in the formation of a new carbon-carbon bond. The product of this step is an alkyne with an ethyl group attached, effectively increasing the size of the molecule.
The final step involves the use of Lindlar's catalyst, which is known for its ability to selectively hydrogenate alkynes to cis alkenes. This means that the triple bond in the product will be converted to a cis double bond, with both large substituents (the ethyl group and the original alkyne) positioned on the same side of the double bond. The outcome of this multistep synthesis is a cis alkene with an ethyl group, showcasing a common synthetic pathway in organic chemistry.