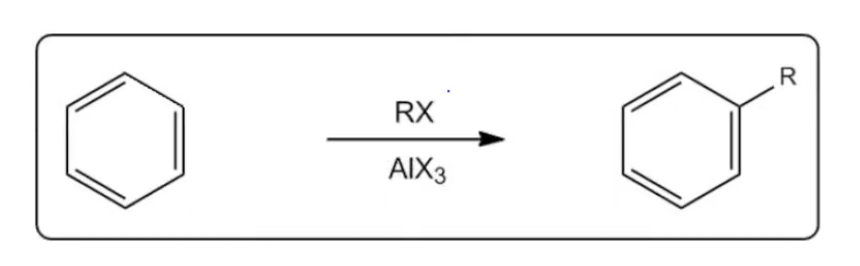
Friedel-Crafts alkylation is a significant reaction in organic chemistry that involves the interaction of an alkyl halide with a strong Lewis acid, typically aluminum trichloride (AlCl3). This reaction generates a potent electrophile, specifically a carbocation, which is crucial for the subsequent electrophilic aromatic substitution (EAS) mechanism. Carbocations are characterized by their electron-deficient nature due to an empty p orbital, making them highly reactive. However, they are also prone to rearrangements, particularly when primary carbocations are formed, as these are unstable and energetically unfavorable.
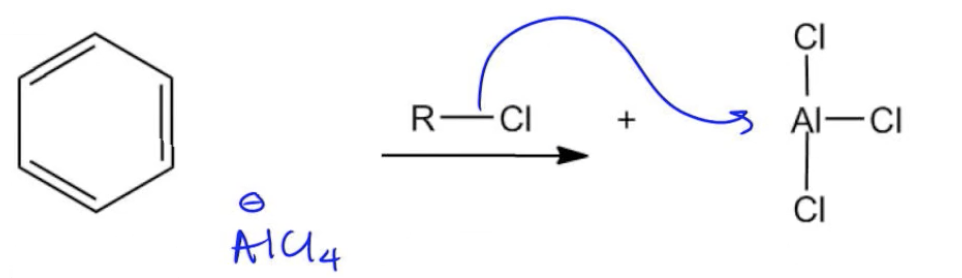
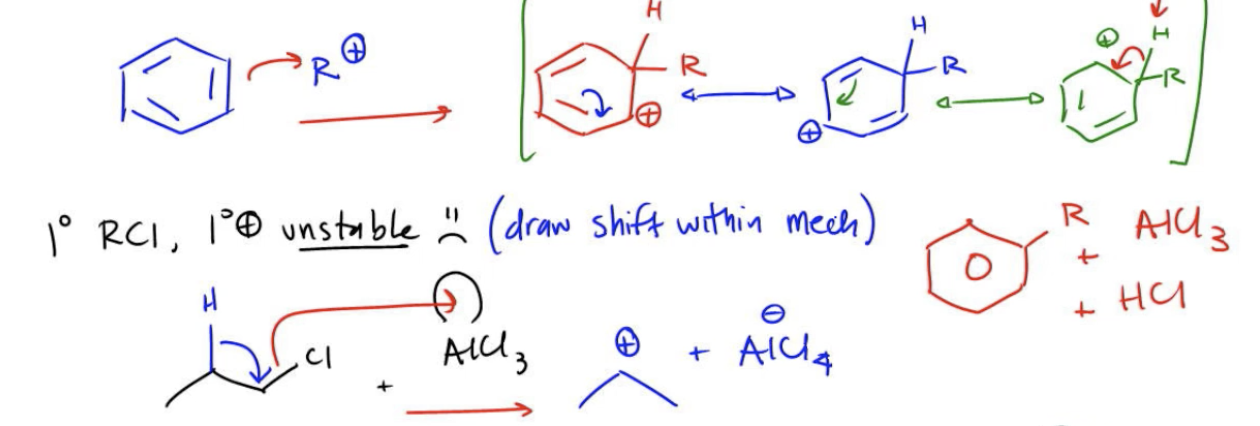
The mechanism begins with the formation of the carbocation. The alkyl halide donates electrons from the carbon-halogen bond to the Lewis acid, resulting in the generation of a carbocation (R+) and a complex with the Lewis acid (AlCl4-). The benzene ring then attacks the carbocation, leading to the formation of a sigma complex, which is a resonance-stabilized intermediate. This complex retains a positive charge, which can be represented by various resonance structures.
In the final step, the negatively charged Lewis acid complex (AlCl4-) facilitates the elimination of a proton from the sigma complex, regenerating the Lewis acid catalyst (AlCl3) and producing HCl as a byproduct. This showcases the catalytic nature of the Lewis acid in the reaction.
When dealing with primary alkyl halides, the mechanism requires careful consideration due to the instability of primary carbocations. If a primary carbocation is formed, it is essential to illustrate a carbocation shift to a more stable secondary carbocation within the same mechanism. This shift allows the reaction to proceed with a more stable electrophile, ensuring that the reaction is feasible and efficient. Overall, understanding the nuances of carbocation stability and rearrangements is critical for successfully predicting the outcomes of Friedel-Crafts alkylation reactions.