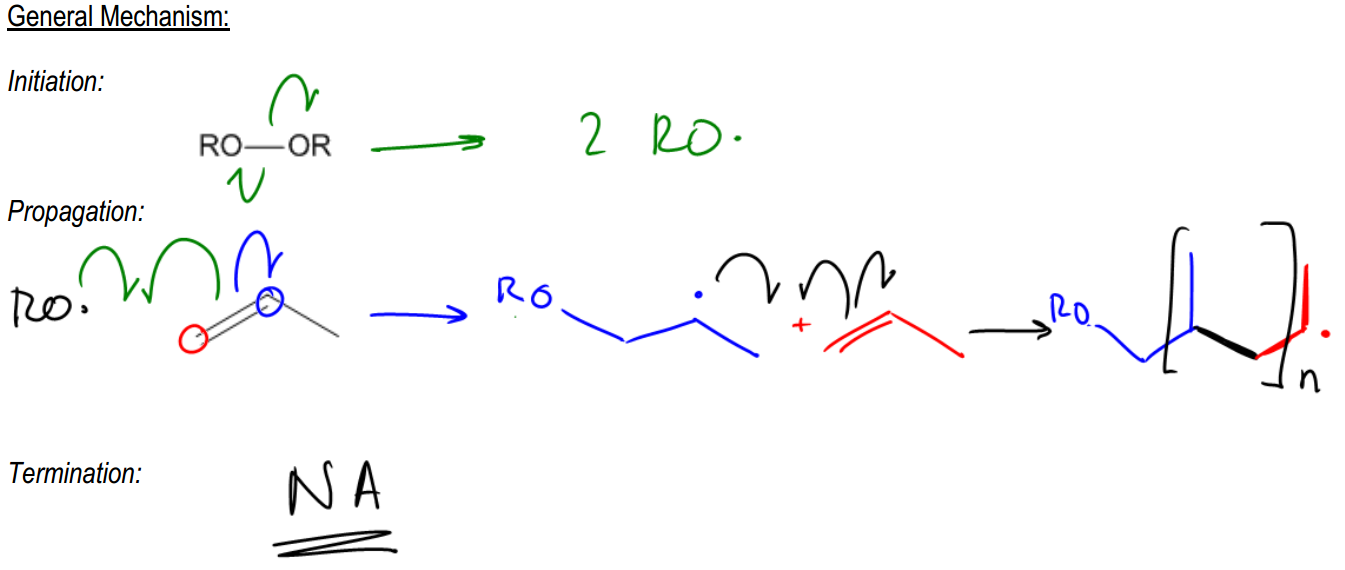
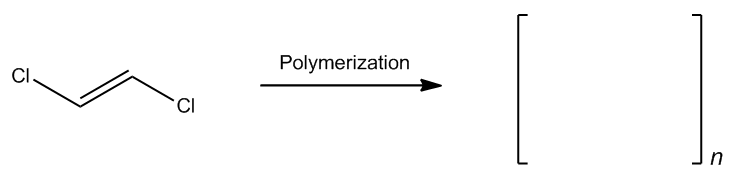
Radical polymerization is a fascinating process that occurs when radicals interact with alkenes in excess, leading to a continuous chain reaction known as polymerization. This reaction can propagate indefinitely, resulting in long hydrocarbon chains that can consist of thousands of repeating units. Such chains are the foundation of many synthetic materials, including plastics, which are derived from petroleum through this method.
In the context of radical polymerization, a common starting material is propylene, a byproduct of petroleum extraction. When a radical initiator, such as a radical species, is introduced, it triggers the polymerization process. The general formula for a polymer can be expressed as CnH2n, where n represents the number of repeating units in the polymer chain. Each repeating unit typically consists of two carbon atoms connected by a double bond, along with additional hydrogen atoms that complete the structure.
For example, in polypropylene, the repeating unit includes two carbon atoms and a methyl group (–CH3) attached to one of the carbons. This structure allows for the formation of extensive chains that can be utilized in various applications, such as astroturf, car tires, and ropes. The ability of these polymers to link and cross-link is what gives rise to the diverse range of synthetic materials we encounter in everyday life.
Overall, the process of radical polymerization not only highlights the significance of alkenes and radicals in chemical reactions but also underscores the vital role of petroleum in producing essential materials that shape modern society.