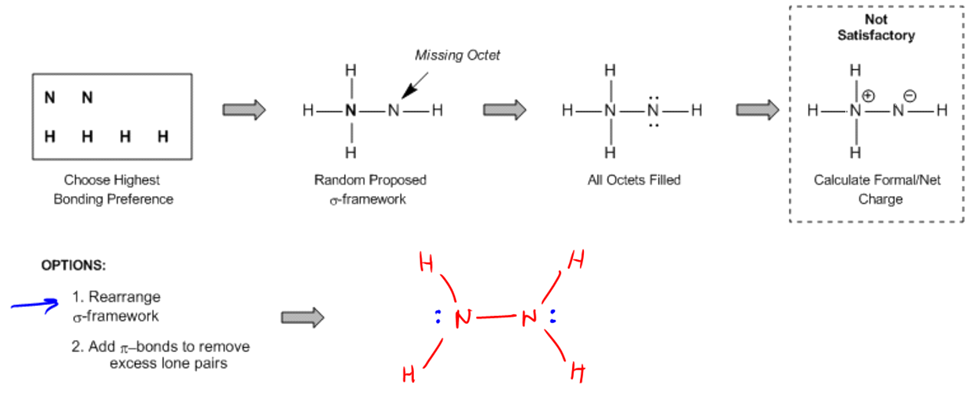
Lewis structures are essential tools for representing molecules, as they explicitly illustrate all atoms, bonds, and lone pairs. Understanding how to construct these structures is crucial, especially when applying knowledge of the octet rule and bonding preferences. To create a Lewis structure from a molecular formula, begin by identifying the atom with the highest bonding preference to place in the center. This atom is typically the most electropositive, meaning it is furthest from fluorine on the periodic table. For instance, nitrogen, which prefers to form three bonds, would be placed in the center over oxygen, which prefers two bonds.
Once the central atom is established, propose a sigma bond framework. This initial proposal may require some trial and error, so it’s important to approach it with flexibility. If two atoms have the same bonding preference, such as sulfur and oxygen, the larger atom (sulfur) should be placed in the center. After establishing the framework, complete the octets of all atoms by adding lone pairs. This step often results in a structure that appears densely packed with dots, representing the lone pairs.
Next, calculate the theoretical number of valence electrons for the molecule and compare it to the actual number of electrons represented in the Lewis structure. The difference between these two values, known as the electron difference, guides further adjustments. If there are excess electrons (a positive electron difference), double bonds may need to be formed to reduce the number of lone pairs. Conversely, if there are too few electrons, additional lone pairs should be added to satisfy the theoretical valence requirements.
However, with a solid understanding of organic chemistry concepts, particularly bonding preferences and formal charges, the process can be simplified. Instead of performing detailed calculations, you can rely on these principles to determine the most stable Lewis structure efficiently. This approach streamlines the process, allowing for quicker and more intuitive construction of Lewis structures without extensive mathematical adjustments.