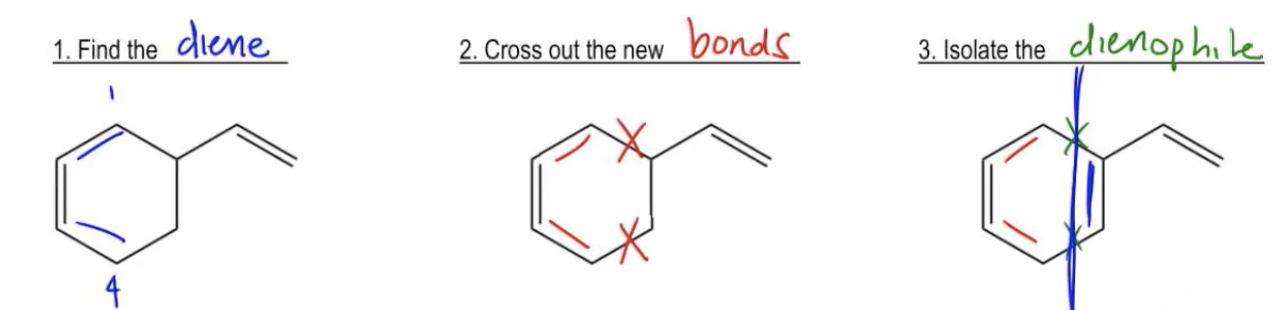
The Diels-Alder reaction is a crucial organic chemistry process that involves the cycloaddition of a diene and a dienophile to form a six-membered ring. Understanding how to perform a retro-synthesis of this reaction is essential for solving problems where the final product is given, and you need to identify the original diene and dienophile.
To approach a Diels-Alder retro-synthesis, start by identifying the new double bond formed in the product. This double bond serves as a landmark, indicating the positions of the original diene. For instance, if the new double bond is between the second and third carbons of the product, these positions correspond to the diene's structure. Label these carbons as 2 and 3, and deduce that the diene must have been present in the original structure.
Next, recognize that the diene forms two new bonds with the dienophile during the reaction. To reverse this process, you will need to eliminate these new bonds from the product structure. Cross out the atoms or groups that represent these new bonds, which will help isolate the dienophile. The remaining structure will give you the diene and the dienophile.
For example, if you identify a diene structure and cross out the new bonds, you can visualize the dienophile by looking at what remains. Often, the dienophile will also have a double bond, which is necessary for the reaction to occur. This systematic approach allows you to deduce the original components of the Diels-Alder reaction effectively.
In summary, mastering the retro-synthesis of Diels-Alder reactions involves recognizing the new double bond, identifying the diene, removing the new bonds to isolate the dienophile, and ensuring that the dienophile has the necessary double bond for the reaction. Practicing this technique with various examples will enhance your understanding and proficiency in organic synthesis.