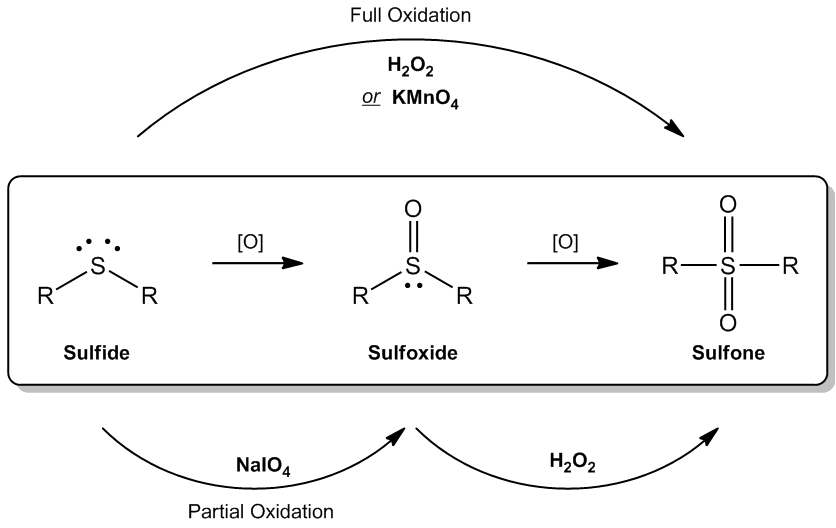
Oxidation reactions involve the increase in the oxygen content of a molecule, which can occur through the formation of additional bonds to oxygen rather than the direct addition of oxygen itself. Understanding this concept is crucial when exploring the oxidation of sulfides, which are compounds containing sulfur with no oxygen atoms initially bonded to it.
As we examine the oxidation process, it is important to recognize that sulfides start with a sulfur atom that has zero bonds to oxygen. During oxidation, the number of bonds to oxygen increases, leading to the formation of various oxidized sulfur species. This pattern illustrates the fundamental principle of oxidation: as a molecule is oxidized, it gains more oxygen bonds, transitioning from a sulfide to more oxidized forms such as sulfones or sulfates.
In summary, the oxidation of sulfides is characterized by the transformation of sulfur from a state with no oxygen bonds to one where it is increasingly bonded to oxygen, reflecting the broader definition of oxidation in chemical reactions.