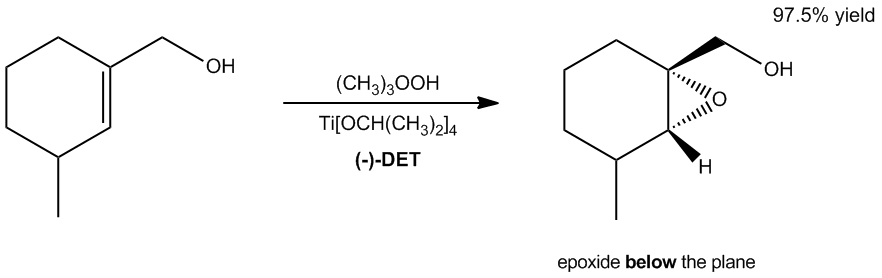
The Sharpless Asymmetric Epoxidation is a significant reaction in organic chemistry, particularly known for its enantioselectivity, which allows for the preferential formation of one enantiomer over another. This reaction specifically targets allyl alcohols, characterized by the presence of a CH2 group adjacent to a double bond. The process utilizes unique reagents, particularly tartrates, which contain chiral centers that influence the outcome of the epoxidation.
There are three types of tartrates that can be employed in this reaction: the positive (S,S) tartrate, the negative (R,R) tartrate, and the meso (R,S or S,R) tartrate. The positive tartrate, which exhibits a clockwise rotation of polarized light, leads to the formation of an epoxide by attacking the double bond from above. Conversely, the negative tartrate, which rotates light counterclockwise, results in the epoxide forming from below the double bond. The meso tartrate, due to its symmetrical nature, does not exhibit optical activity and thus does not favor either direction of attack, leading to a non-enantioselective outcome with a 50% chance of forming either epoxide.
In summary, the choice of tartrate is crucial in determining the stereochemistry of the resulting epoxide. The positive and negative tartrates are utilized to achieve specific enantiomeric products, while the meso variant is generally not used in synthetic applications due to its lack of selectivity. This reaction exemplifies the importance of chirality in organic synthesis and the ability to control the formation of specific stereoisomers through careful selection of reagents.