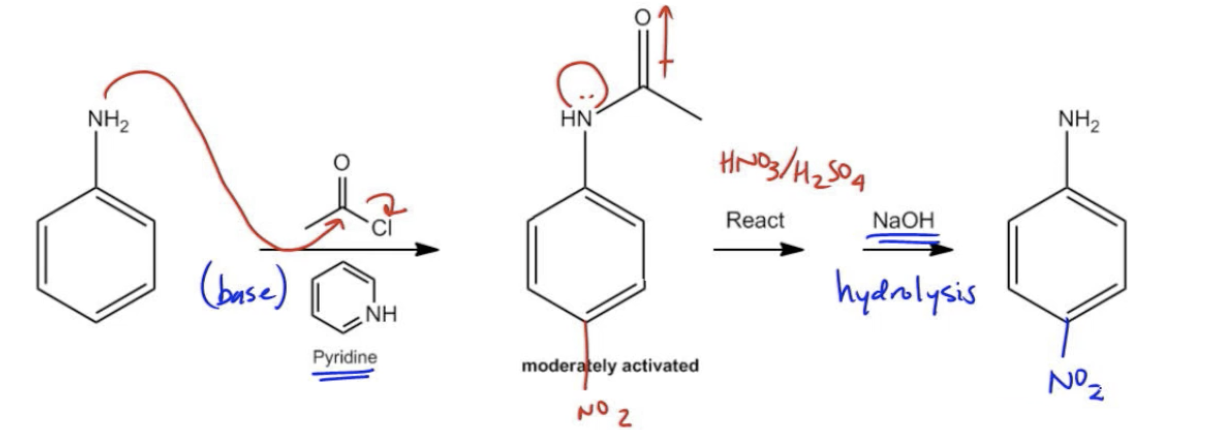
In the context of electrophilic aromatic substitution (EAS) reactions, aniline presents unique challenges due to its highly activating nature. The amino group (-NH2) on aniline is the most activating substituent, which typically directs electrophiles to the ortho and para positions. However, this high reactivity can lead to polysubstitution, where multiple electrophiles react with the aromatic ring, complicating the desired outcome of the reaction.
For instance, when attempting to nitrate aniline using nitric acid and sulfuric acid, one might expect to obtain a mixture of ortho and para nitroaniline products. However, the excessive reactivity of aniline can result in unwanted side reactions, necessitating a protective strategy. To mitigate this issue, a process known as acetylation is employed. Acetylation involves the introduction of an acetyl group (-COCH3) to the nitrogen atom of aniline, which temporarily reduces its reactivity.
The acetylation reaction typically utilizes an acid chloride in the presence of a base, such as pyridine. The mechanism involves the nitrogen atom attacking the carbon of the acid chloride, leading to the formation of the acetylated product while releasing a chloride ion. This modification transforms the aniline from a strongly activated state to a moderately activated state, allowing for controlled EAS reactions without the risk of polysubstitution.
Once the desired electrophilic substitution has occurred, the acetyl group can be removed through hydrolysis, restoring the original aniline structure but now with the new substituent in place. This hydrolysis process is facilitated by a base, which cleaves the acetyl group from the nitrogen. It is crucial to remember that whenever working with aniline in EAS reactions, the protection step via acetylation is essential to prevent unwanted reactions and ensure successful synthesis of the target compounds.
In summary, understanding the reactivity of aniline and the necessity of protection through acetylation is vital for successfully conducting EAS reactions. This approach allows chemists to navigate the challenges posed by highly activated aromatic systems while achieving the desired synthetic outcomes.