Understanding the arrangement of double bonds is crucial in organic chemistry, particularly when it comes to naming and identifying isomers. Double bonds restrict rotation due to the overlap of p-orbitals, leading to distinct spatial arrangements known as cis and trans configurations. These terms are used to describe the relative positioning of substituents attached to the carbon atoms involved in the double bond.
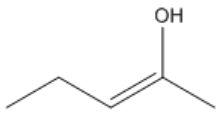
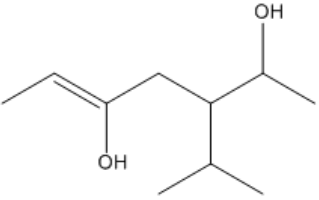
When two groups are oriented on the same side of the double bond, the configuration is termed cis. Conversely, if the groups are on opposite sides, the configuration is referred to as trans. This distinction is essential because it affects the physical properties and reactivity of the molecules. For example, in the case of 2-butene, the cis isomer has both substituents on the same side of the double bond, while the trans isomer has them on opposite sides. This can be visualized by drawing a dotted line through the double bond, which acts as a 'fence' to determine the relative positions of the groups.
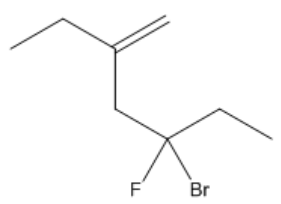
It is important to note that cis and trans nomenclature applies only to groups attached to different carbon atoms. If two substituents are on the same carbon, they do not exhibit cis or trans relationships and are named differently. The concept of stereoisomers further expands on this idea, indicating that while the connectivity of atoms remains the same, their spatial arrangement differs, leading to variations in properties.
In summary, recognizing the significance of cis and trans configurations in double bonds is vital for understanding molecular behavior and properties. The inability to rotate around double bonds creates unique isomers that can have markedly different characteristics, making this knowledge essential for studying organic compounds.