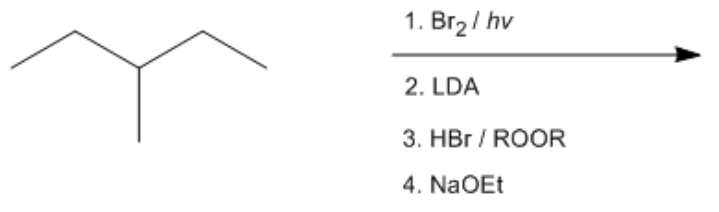
In organic chemistry, radical halogenation, specifically radical bromination, serves as a foundational reaction for transforming alkanes. Starting with an alkane, bromine is introduced at the tertiary position, which is crucial for the subsequent steps. The use of LDA (Lithium diisopropylamide) with a tertiary alkyl halide leads to a Hoffman elimination, a reaction characterized by the formation of less substituted alkenes. This is significant because it emphasizes the preference for less sterically hindered products in certain elimination reactions.
When considering the formation of double bonds, the choice of beta hydrogens is essential. In a Hoffman elimination, the double bond is formed at the less substituted position, yielding the major product. Following this, the introduction of HBr in the presence of peroxides results in radical hydrohalogenation, which is notable for producing anti-Markovnikov alkyl halides. This means that bromine will add to the less substituted carbon, further diversifying the product.
The final step involves the use of sodium ethoxide (NaOEt), a strong nucleophile. The flowchart approach to determining the reaction mechanism is vital here. Since NaOEt is negatively charged and not bulky, and given that the alkyl halide is primary, the reaction favors an SN2 mechanism. This results in a backside attack, leading to the formation of an ether as the final product. The transformation from an alkane to an ether through these four steps illustrates the power of organic synthesis, showcasing how simple alkanes can be converted into more functionalized molecules.
Overall, this process highlights key concepts in organic chemistry, including radical reactions, elimination mechanisms, and nucleophilic substitutions, all of which are fundamental for understanding the reactivity and transformation of organic compounds.