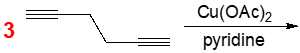The Eglinton reaction is a specific type of coupling reaction that involves the combination of two identical terminal alkynes, facilitated by a copper catalyst and a base, typically pyridine. This reaction is notable for its focus on identical alkynes to prevent the formation of a mixture of products, ensuring a more predictable outcome.
In the Eglinton coupling, the primary driving forces are the formation of conjugated products, which enhance stability due to increased resonance, and the unique mechanism that relies on radical intermediates rather than a traditional catalytic cycle. The general setup includes two identical alkynes, denoted as alkyne 1 and alkyne 2, along with copper(I) and copper(II) catalysts, and pyridine as the base. Pyridine, a nitrogen-containing aromatic compound, plays a crucial role in the reaction.
During the reaction, hydrogen atoms are lost from both terminal alkynes, allowing the remaining fragments to combine and form a bialkene product. The identical nature of the alkynes means that their substituents, represented as R groups, can vary; they may include vinyl, aryl, or alkyl groups. This flexibility in substituent choice allows for a range of potential products while maintaining the integrity of the reaction mechanism.
Understanding the Eglinton reaction is essential for grasping the broader concepts of organic synthesis and the importance of reaction conditions in determining product outcomes. The next step involves exploring the detailed mechanism to fully appreciate how the reaction proceeds and the factors influencing the final product formation.