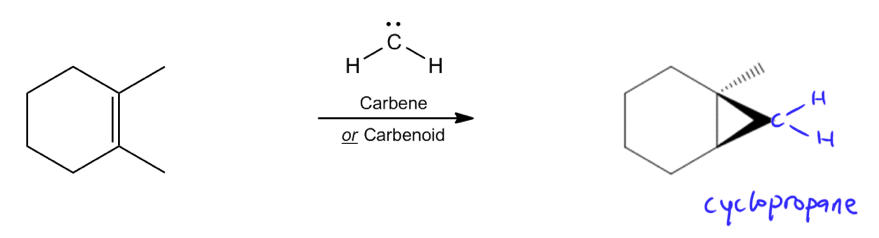
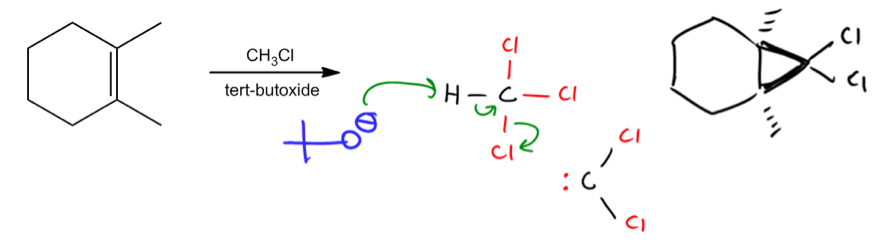
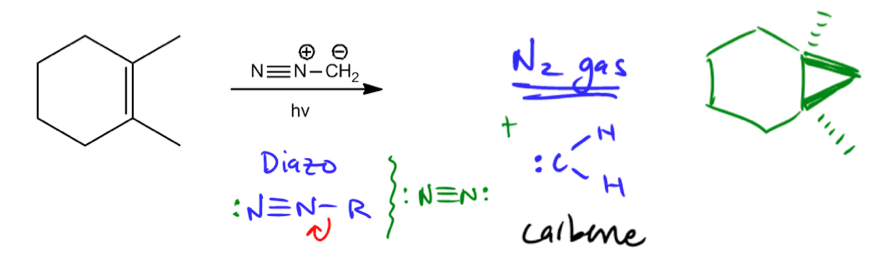
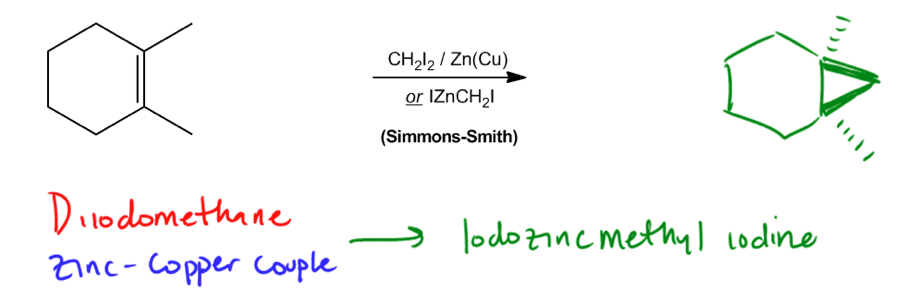
Cyclopropanation is a type of addition reaction that occurs when a double bond interacts with a reactive species known as a carbene or a carbonoid. The primary outcome of this reaction is the formation of a cyclopropane, which involves the addition of a methylene group (–CH2) to the double bond. This process effectively transforms the double bond into a three-membered ring structure.
Carbenes, despite having a formal charge of zero, are highly reactive intermediates. Their reactivity stems from their violation of the octet rule; carbenes possess only six electrons in their valence shell, leaving them craving two additional electrons to achieve a stable octet. This electron deficiency makes them eager to react with double bonds, leading to the formation of cyclopropane products.
In summary, the cyclopropanation reaction is a valuable synthetic tool in organic chemistry, allowing for the efficient conversion of alkenes into cyclopropanes through the action of carbenes or carbonoids. Understanding the nature of these reactive intermediates is crucial for predicting the outcomes of cyclopropanation reactions and their applications in various chemical syntheses.