Conjugation is a concept in organic chemistry that occurs when three or more atoms capable of resonance are adjacent to each other, allowing their orbitals to overlap. This overlapping facilitates the delocalization of electrons across the molecule, which enhances its stability. While the terms "resonance" and "conjugation" are often used interchangeably, it is important to note that resonance refers to the action of electrons delocalizing, while conjugation describes the structural ability of a molecule to resonate.
One of the key implications of conjugation is that it creates an "electron highway," enabling electrons to move freely from one part of the molecule to another. This delocalization contributes to the overall stability of the molecule and influences its chemical reactivity. As a result, conjugated molecules often exhibit unique properties that differ from their non-conjugated counterparts.
Additionally, the degree of conjugation in a molecule is directly related to its absorption of ultraviolet (UV) light. Specifically, as the level of conjugation increases, the wavelength of light absorbed by the molecule in a UV-Vis spectrometer also increases. This relationship is significant in organic chemistry, as it can be a useful indicator of the extent of conjugation present in a compound.
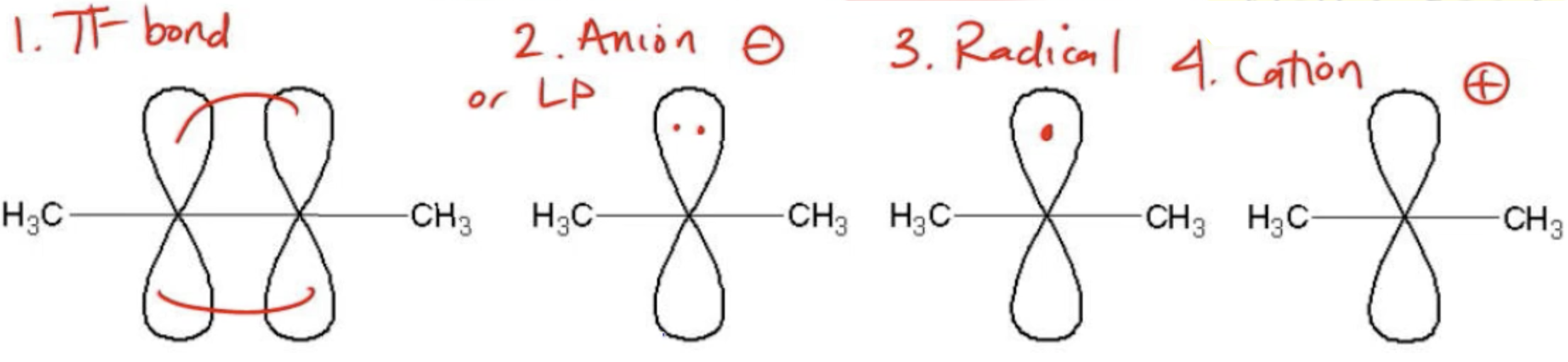
In terms of the types of atoms that can participate in conjugation, pi bonds are essential. Both double bonds (which contain one pi bond) and triple bonds (which contain two pi bonds) can resonate. Furthermore, atoms with lone pairs, anions, radicals, and cations can also contribute to conjugation. Each of these species can participate in resonance, allowing for the necessary overlap of orbitals. Therefore, a conjugated system typically requires a combination of these atoms in a sequential arrangement to facilitate resonance.
To identify whether a molecule is in a conjugated state, one must look for the presence of these resonating atoms in a continuous chain. Understanding these principles will aid in recognizing conjugated systems and predicting their chemical behavior.