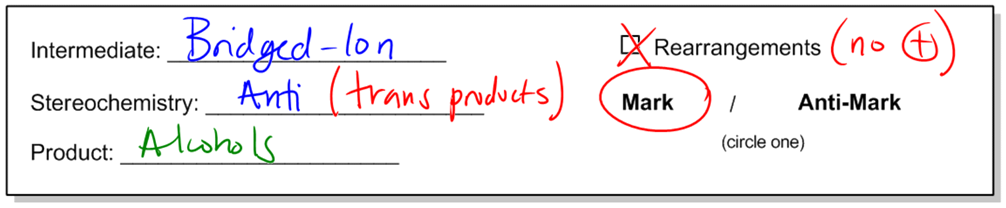
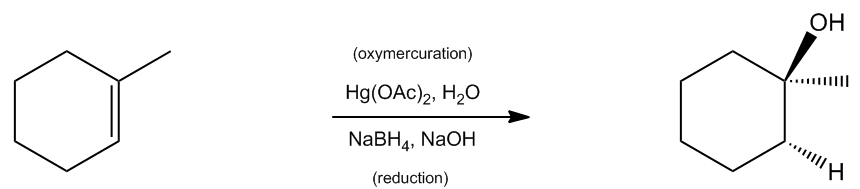
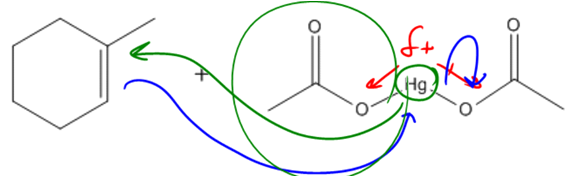
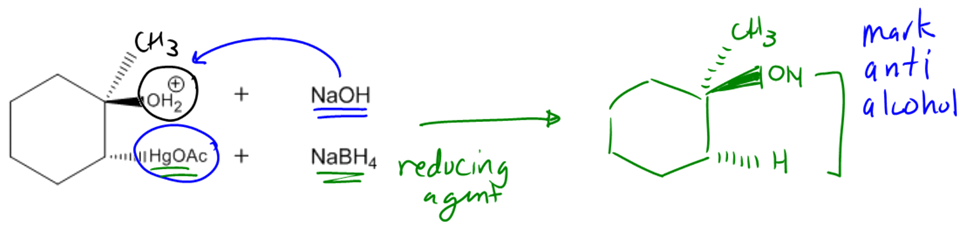
In organic chemistry, the addition of alcohols to double bonds can be achieved through various methods, one of which is known as oxymercuration-demercuration, often abbreviated as oxymerc. This reaction involves two main steps: the oxymercuration step and the demercuration (or reduction) step. The reagents used in the first step include a mercury compound with two acetyl groups (denoted as Hg(OAc)2) and water. The presence of mercury in the reagent is a key indicator that the reaction is oxymercuration.
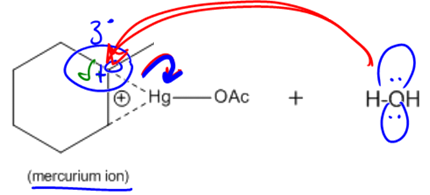
During the oxymercuration step, a bridged ion intermediate is formed instead of a carbocation, which is significant for predicting the stereochemistry of the final product. The stereochemistry resulting from this reaction is characterized as anti, meaning that the alcohol and hydrogen added to the double bond will be trans to each other in the final product. This is an important aspect to remember, as it influences the spatial arrangement of the substituents.
Following the oxymercuration step, the reaction proceeds to the demercuration step, where sodium borohydride (NaBH4) and a base such as sodium hydroxide (NaOH) are used. This step effectively removes the mercury and replaces it with a hydrogen atom, resulting in the formation of an alcohol. Notably, this reaction adheres to Markovnikov's rule, which states that the more stable carbocation will receive the more substituted alcohol. In the case of oxymercuration, even though a carbocation is not formed, the principle still applies, ensuring that the alcohol is added to the more substituted carbon of the double bond.
One of the advantages of the oxymercuration-demercuration method is that it does not involve carbocation rearrangements, which can complicate product formation in other reactions. Therefore, the products can be predicted with greater certainty based on the initial structure of the alkene and the reagents used. The final product will exhibit Markovnikov orientation with anti stereochemistry, resulting in a stable and predictable outcome.