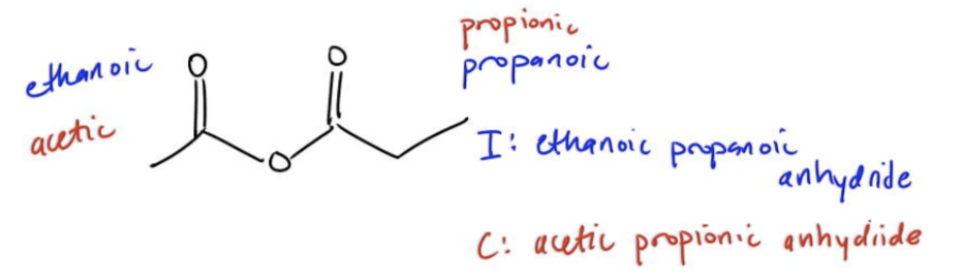
Anhydrides are a specific type of functional group that are derived from carboxylic acids. Essentially, an anhydride can be visualized as a combination of two carboxylic acids, where the formation occurs through the condensation of these acids, resulting in the loss of a water molecule. Structurally, an anhydride can be thought of as a dicarbonyl compound with an oxygen atom in the middle, represented as R(C=O)₂O, where R denotes the carbon chains from the carboxylic acids.
When it comes to naming anhydrides, the process is straightforward. You take the names of the two carboxylic acids involved, arrange them in alphabetical order, and replace the suffix "acid" with "anhydride." For example, if you have ethanoic acid and propanoic acid, the IUPAC name for the anhydride would be ethanoic propanoic anhydride. In common naming conventions, this would be referred to as acetic propionic anhydride. It is crucial to memorize the common names of the first five carboxylic acids, as they frequently appear in various derivatives.
In cases where both R groups in the anhydride are identical, the naming simplifies. Instead of referring to two different acids, you can simply use the name of the acid followed by "anhydride." For instance, if both sides of the anhydride are derived from acetic acid, it would be named acetic anhydride.
Understanding the structure and naming conventions of anhydrides is essential for mastering organic chemistry, as these compounds play significant roles in various chemical reactions and applications.