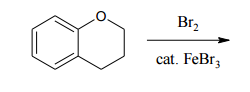
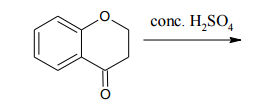
When dealing with polysubstituted benzene compounds, understanding the placement of additional substituents becomes more complex due to the presence of multiple groups. The introduction of two or more substituents leads to considerations of steric effects and directing effects, which significantly influence the outcome of electrophilic aromatic substitution (EAS) reactions.
Steric effects arise when substituents hinder the reactivity of certain positions on the benzene ring. For instance, positions located between two bulky groups may become unreactive due to steric hindrance. This means that these sites will be the least favorable for further substitution, as they are surrounded by sterically hindered groups, making them less accessible for electrophiles.
In addition to steric effects, the directing effects of substituents must be analyzed. Each substituent can either activate or deactivate the ring towards further substitution, and their influence can be synergistic or competitive. Synergistic directing groups work together to direct incoming electrophiles to the same position, resulting in a higher yield of the desired product. For example, if both a nitro group and an aldehyde group direct towards the same meta position, the reaction will favor that site, leading to a high yield of the product.
Conversely, competitive directing groups may direct incoming electrophiles to different positions, leading to a mixture of products and lower overall yields. In a scenario where a nitro group and an aldehyde group are positioned such that they direct to different meta positions, both products will form, but the yield of each will depend on the relative activating strength of the groups involved. The strongest activator will typically dictate the major product, while the weaker one will yield a minor product.
In terms of reactivity, it is essential to remember that nitro groups are strong deactivators and weak activators, while carbonyl groups (like aldehydes) are moderately deactivating but can be more active than nitro groups in certain contexts. This means that when determining the major product in a competitive scenario, the relative activity of the substituents is crucial. The product formed at the position directed by the stronger activator will be in higher yield, while the other will be in lower yield.
Overall, understanding the interplay of steric and directing effects is vital for predicting the outcomes of EAS reactions in polysubstituted benzene compounds. This knowledge allows chemists to navigate the complexities of substitution patterns effectively, leading to desired synthetic outcomes.