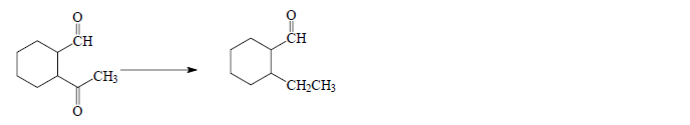Acetals serve as effective protecting groups in organic synthesis, particularly for sensitive carbonyl compounds like aldehydes and ketones. The process of forming an acetal involves the reaction of a carbonyl with an alcohol in the presence of an acid catalyst, typically represented as H+. When a cyclic diol is used, the result is a cyclic acetal, which is significantly less reactive than the original carbonyl compound.
The key distinction between carbonyls and acetals lies in their reactivity. Carbonyls possess a highly reactive partial positive charge on the pi bond, making them susceptible to various reactions, including hydrolysis with water. In contrast, acetals have dipoles located on sigma bonds, which are much more stable and difficult to break. This stability makes acetals comparable in reactivity to ethers, which are known for their low reactivity and primarily undergo combustion.
In synthetic applications, the use of acetals as protecting groups is advantageous. By converting a carbonyl into an acetal, chemists can shield the reactive carbonyl from unwanted reactions with other reagents during subsequent steps in a synthesis. Once the desired reactions are completed, the acetal can be hydrolyzed back to the original carbonyl, allowing for the recovery of the sensitive functional group.
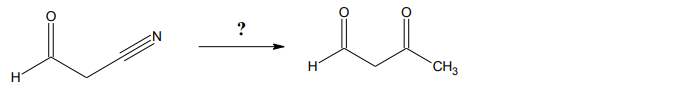
To illustrate this concept, consider a transformation involving two specific molecules. The synthesis from the first molecule to the second is not achievable in a single step; instead, it requires a sequence of reactions. This highlights the importance of strategic planning in organic synthesis, where the use of protecting groups like acetals can facilitate complex transformations while preserving the integrity of reactive functional groups.