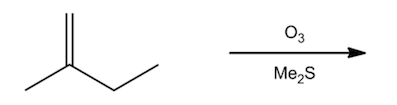Ozonolysis is a type of weak oxidative cleavage where we cleave alkenes (double bonds) into either ketones, aldehydes or carboxylic acid using ozone.
Topic: Weak Oxidative Cleavage
The History of Ozonolysis:
Ozonolysis, or “oxidative cleavage” originated in the 1800’s with its inventor, Christian Friedrich Schönbein. The reaction also is attributed to Carl Dietrich Harries, therefore you may hear this reaction termed “Harries ozonolysis”.

Ozone Structure
It is in simplest terms using ozone, or O3 (the structure is shown above with correct formal charges) to cleave carbon-carbon double bonds (C=C) to produce various carbonyls.
2 Pathways:
1. Aldehydes (CHO), and ketones (CH3COCH3) can be formed through reductive workup. This refers to what is shown under the arrow below. Me2S is also DMS and stands for dimethyl sulfide.

Ozonolysis Retrosynthesis
2. Carboxylic acid (COOH, CO2H) can also be formed as well except it is through an oxidative workup step instead. H2O2 (hydrogen peroxide) is the reagent to look out for because as seen below it turns:
- Ends of alkenes with 1 –H ≠
Aldehydes but Carboxylic Acids instead

Ozonolysis with Oxidative Workup
Answer from above:

Di-substituted alkene
How Does Ozonolysis Work?
Now we will discuss the specifics of the mechanism of ozonolysis.
A. Reagents:
Commonly seen is the use of O3, which is ozone (structure shown above), in the presence of a reducing agent such as dimethyl sulfide (DMS, Me2S, S(CH3)2) or zinc & acetic acid (Zn/HOAc).
Therefore a full reaction may look like this:

Ozonolysis Reaction
Now as we said, instead of DMS under the arrow you may also see zinc and acetic acid. Beware of the other ways “acetic acid” can be written in addition to HOAc. They are: [AcOH, CH3COOH, CH3CO2H]
This type of mechanism is referred to as ozonolysis with reductive workup.
In case you need to know:
After the reduction takes place our Zn or DMS will attach to the remaining third oxygen from our O3 that is not seen in our final product. Because of this, along with our carbonyl products we will also find DMSO (dimethyl sulfoxide) or ZnO (zinc oxide) produced as well.

Ozonolysis Mechanism
DMSO is simply the blue structure you see at the top right of the image. A couple other intermediates you may see are the molozonide and the ozonide which we will discuss below.
B. Reaction Mechanism and Intermediates:
When the reaction takes place, the first intermediate you will see is called your molozonide. It is a cyclic structure containing 3 oxygen atoms connected in a row, also referred to as a 1,2,3-trioxolane.

Molozonide Intermediate
This will be your unstable intermediate which will further undergo a rearrangement to form your ozonide. An ozonide is more stable than the molozonide, however it still exhibits a fair amount of instability.

Ozonide Intermediate
These are not isolated either and will further be reduced or oxidized depending on if you follow the “reductive workup” pathway or the “oxidative workup” pathway.
*Rudolf Criegee proposed the mechanism therefore if you hear words such as the ‘Criegee zwitterion’ or ‘Criegee intermediate’ that is why! It is referring to an intermediate that appears between the molozonide and the ozonide.

Criegee Intermediate
C. Products:
The products of ozonolysis will vary depending on two things:
1) The R groups that are attached to the alkene:
1. Ends of alkenes with –R groups on both sides = Ketones
2. Ends of alkenes with 1 –H = Aldehydes
3. Ends of alkenes with 2 –Hs (yielding single carbon fragments) = Formaldehyde
2) Instead of a “reductive workup” with either zinc (Zn) and acetic acid (HOAc), we use an “oxidative workup” with hydrogen peroxide (H2O2).

Ozonolysis with Oxidative Workup of Cyclic Alkene
What this will do is alter our products so that any aldehydes (CHO) that were formed in our cleavage step will be oxidized into carboxylic acids (COOH).
Question: Can you guess what the product will be here? I’ll give you a hint. Cut across the double bond and add in the missing hydrogen on the red C. Then use the rule above to predict the final product. Scroll down for the answer :)
Special Ozonolysis Cases:
# 1 - Fragments
- You may sometimes hear the products of this reaction referred to as “fragments”. All this is referring to is the different molecules that were formed when we broke apart our original C-C double bond.
- For example if 2 aldehydes (CHO) were your products, you could say that 2 aldehyde fragments were formed while performing an reductive cleavage.
- This word is more commonly used when multiple alkenes are cleaved.
#2 - Pyridine
- Pyridine is commonly seen as a buffer when any kind of acid is generated in the reaction.
#3 - Alcohols
- If NaBH4 (sodium borohydride) is used as the reagent during the reductive workup we will yield alcohols instead of aldehydes & ketones as seen when Zn, or DMS is used.

Reduction with Sodium Borohydride
# 4 - Dichloromethane
- Dichloromethane (CH2Cl2) in particular may be seen as a solvent to help with cleavage of the ozonide intermediate to yield our product.
# 5 - Triphenylphosphine
- Triphenylphosphine (PPH3, PH3P) is one of the other reagents used, just as we learned with Zn and DMS to achieve our product in a reductive workup mechanism.
Answer to above example with H2O2:

Dicarboxylic Acid
“I’m confused about....”
a. Which step of the mechanism is sometimes known as reductive ozonolysis?
- The last step where we go from our ozonide to the product is known as the reductive workup. It is commonly seen more than the oxidative one. Sometimes seen to accomplish this along with DMS & Zn is PPH3 (Triphenylphosphine).
b. What is meant by the word ‘quench’?
- Generally speaking this means to stop the reaction by getting rid of any unreacted reagents, usually with an aqueous solution.
c. What are some of the reaction conditions? (Mainly for lab)
- Temperature wise it is suggested to run this reaction at around -78 ̊C.
d. Are they any indicators I should know about that are used in this reaction?
- Yes, blue usually means you are done reacting with the alkene. Also, violet can be seen along with Sudan Red III which is specifically used for special cases where multiple alkenes can react at different rates.
Just a Quick Recap:
Remember, if you see O3 + alkene these are conditions for the ozonolysis mechanism. Your product will be some form of a carbonyl, either an aldehyde, ketone, or carboxylic acid if using H2O2.