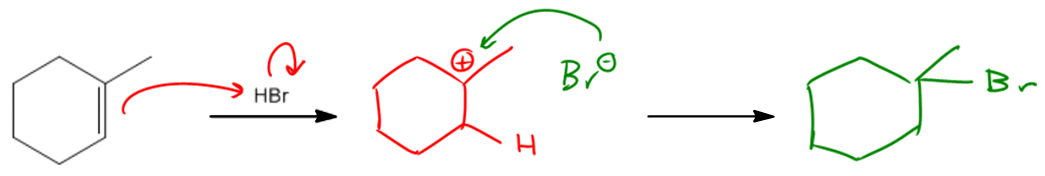
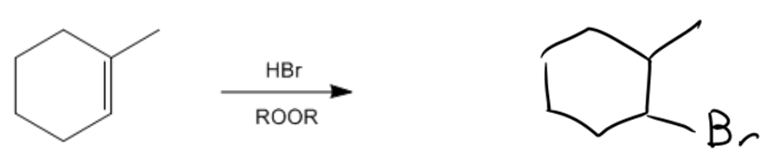
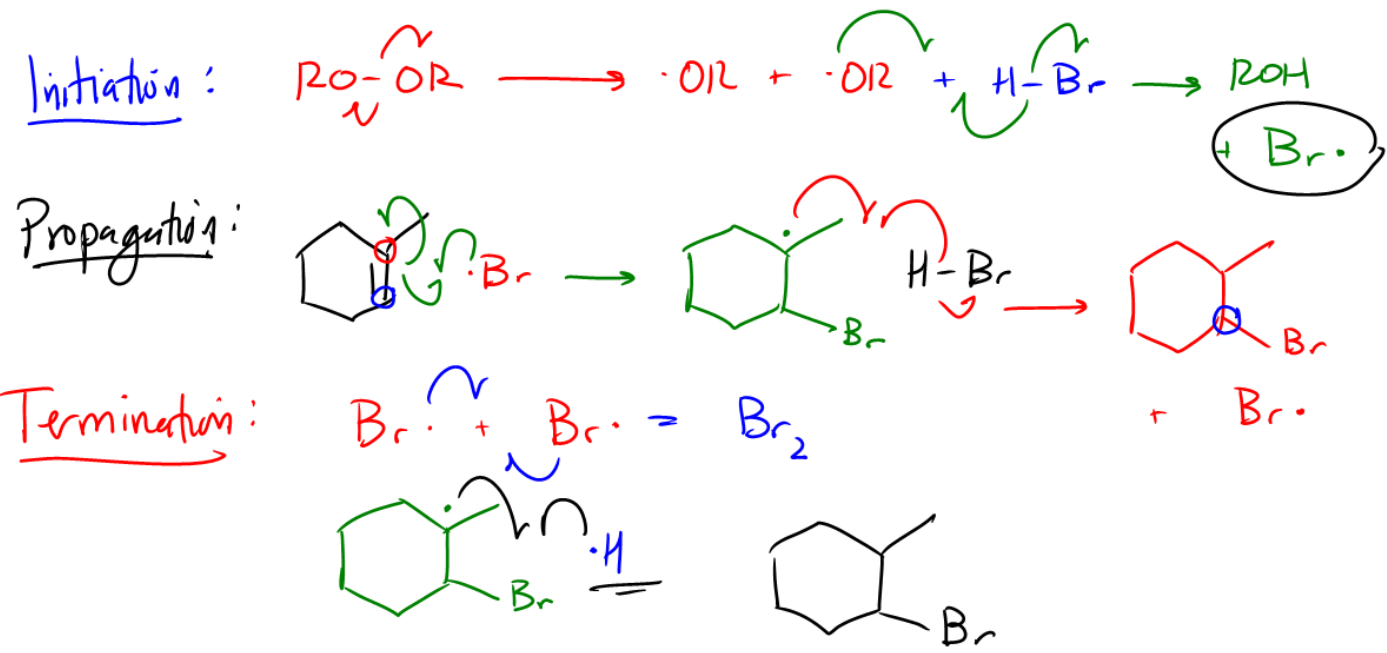
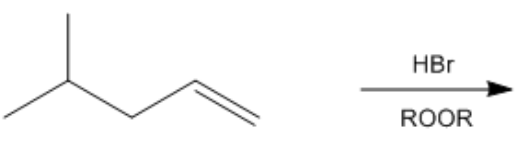
In organic chemistry, the presence of a radical initiator, such as peroxide, can significantly alter the course of a reaction involving alkenes and hydrogen bromide (HBr). When peroxide is introduced, the reaction transitions from a carbocation-mediated mechanism to a radical-mediated mechanism, which involves three key steps: initiation, propagation, and termination.
The initiation step begins with the generation of radicals from the peroxide. This process produces two alkoxy radicals (RO•). One of these radicals then reacts with HBr, resulting in the formation of a bromine radical (Br•) and an alcohol (ROH). This bromine radical is crucial as it serves as the target radical for the subsequent propagation step.
During the propagation phase, the bromine radical interacts with the double bond of the alkene. Instead of abstracting a hydrogen atom, the bromine radical can directly react with the double bond, leading to the formation of a new bond. The key decision in this step is determining where the bromine will attach. The bromine will preferentially bond to the less substituted carbon atom of the double bond, which results in the formation of a more stable radical intermediate at the more substituted carbon. This behavior is characteristic of anti-Markovnikov addition, where the bromine attaches to the least substituted position of the alkene.
As the reaction progresses, the radical intermediate can further react with HBr, ultimately yielding an alkyl bromide product. This product formation is significant because it highlights the unique nature of radical reactions, distinguishing them from traditional electrophilic additions that follow Markovnikov's rule.
The termination step involves the combination of radicals to form stable products. Common termination reactions include the pairing of two bromine radicals to form Br2 or the reaction of the radical intermediate with another radical species. While there are multiple possible termination pathways, the focus is typically on the most favorable outcomes.
Understanding this radical mechanism is essential, as it represents one of the few reactions in organic chemistry that results in anti-Markovnikov addition. The two primary reactions that exhibit this behavior are the radical addition of HBr and hydroboration-oxidation. Recognizing when a reaction will proceed via a radical mechanism is crucial for predicting product formation in organic synthesis.