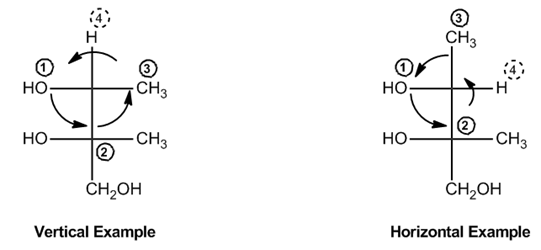
Understanding the determination of absolute configurations in Fischer projections can be simplified using a systematic approach. The key to this method lies in identifying the position of the lowest priority group, which is always designated as group 4. This group can either be positioned vertically or horizontally in the projection.
When the lowest priority group (4) is vertical, the chirality of the molecule is interpreted directly as it appears. To determine the configuration, you trace a path from the highest priority group (1) to the second (2) and then to the third (3). The direction of this path indicates whether the configuration is R (rectus) or S (sinister). If the path is clockwise, the configuration is R; if counterclockwise, it is S.
Conversely, if the lowest priority group (4) is horizontal, the chirality must be flipped. In this case, you still trace the path from 1 to 2 to 3, but the resulting configuration will be the opposite of what you observe. Thus, if the path appears to indicate S, it is actually R, and vice versa.
For example, if you have a chiral center where group 4 is vertical, and you trace the path from 1 to 2 to 3 resulting in a counterclockwise direction, you would conclude that the configuration is S. However, if group 4 is horizontal and the same path indicates S, you would conclude that the actual configuration is R.
This method streamlines the process of determining R and S configurations, especially in complex Fischer projections with multiple chiral centers. By focusing on the position of the lowest priority group, you can quickly ascertain the absolute configuration without the need for swapping groups, making it a valuable technique in stereochemistry.