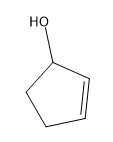
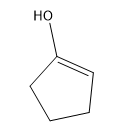
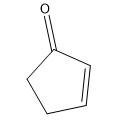
Tautomerization is the name given to the process by which keto and enol forms interconvert. The keto and enol forms are constitutional isomers that reach an equilibrium in acidic or basic conditions.
Keto-Enol tautomerization
Tautomerization is possible in both acidic and basic solution. Let’s look at the mechanisms to go from ketone to enol and back to ketone. The keto form is the familiar carbonyl, while the enol form is basically a vinyl alcohol.

Acid-catalyzed mechanism
The first step in the acidic mechanism is protonation. Heads up: H3O+ is the same thing as H2O and H+. Once the protonated carbonyl is formed, the conjugate base will remove the acidic alpha hydrogen. This forms a bond between the carbonyl carbon and the alpha carbon, and the carbonyl’s pi-electrons are kicked onto the oxygen to form the enol.
To go back to the keto form the oxygen reforms the carbonyl, and the C=C bond’s pi-electrons deprotonate the H3O+ to form H2O again. Then the water removes the proton from the oxygen and voila! You’ve got nice C-C and C=O bonds again.

Base-catalyzed mechanism
The base-catalyzed mechanism starts with the deprotonation of the alpha carbon by a base like –OH. This deprotonated enol is called an enolate, and it’s found extensively in condensation reactions. The anionic oxygen grabs the hydrogen from the conjugate acid, and now we’ve got ourselves an enol.
To go back to the keto form, the alcohol is deprotonated. Then the anionic oxygen kicks a lone pair down to reform the carbonyl, and the C=C bond’s pi-electrons are used to deprotonate the H2O to form –OH again.
Equilibrium direction

Keto-enol equilibrium
In general, the keto form predominates in equilibrium because it is lower in energy. You can even think about this in terms of an acid-base reaction. Equilibrium direction goes from more acidic to less acidic, so it makes sense that the equilibrium would favor the keto form. An alpha hydrogen’s pka is about 20, and an alcohol’s pka is about 16. The alcohol is about ten thousand times as acidic as the alpha carbon.

Beta-dicarbonyl tautomerization
The equilibrium of beta dicarbonyls will actually shift toward the enol form. Why? The enol form is conjugated from one oxygen to the other though the c-c pi-bond, and it also has that hydrogen attached to one of the oxygens which results in hydrogen bonding to the other oxygen.
Imine-Enamine tautomerization

Imine-enamine tautomerization
Imines and enamines can actually tautomerize just like ketones and enols. Imine-enamine tautomerization actually plays a pretty big role in enamine alkylation through the Stork enamine mechanism.