By definition, functional groups are specific groups of atoms commonly found across molecules all over the universe, that look and behave similarly when exposed to like conditions.
INTRO: WHY DO WE NEED FUNCTIONAL GROUPS?
Functional groups are some of the first terms you learn to recognize in undergraduate organic chemistry- and they happen to be some of the most important. You’ll need many of these later, for example when you study carbohydrates and protein functional groups in biology.
In this post I’m going to teach you how to memorize them based on this chart:

The 17 most Important Functional Groups Chart
Why do we even need functional groups? There are quite literally infinite combinations of molecules found in the universe. We could spend the next 100 years learning the name and reactivity of each molecule separately OR we could try to find some patterns in the chaos.
Functional groups are those patterns.
Specifically, when compared against each other, they share similarities in polarity, acidity, chemical reactivity, etc.
HEADS UP: For this topic, I’m going to assume you already know how to represent molecules in skeletal (bond line/line angle) form. Meaning I’ll be dropping all hydrogens and lone pairs. If you still need help with understanding these, feel free to watch my videos on skeletal structure.
PART 1: MEMORIZING THE 17 MOST IMPORTANT FUNCTIONAL GROUPS
1. Hydrocarbons: Starting with the Backbone
Alkanes (C-C single covalent bonds) are the skeleton of organic chemistry. For that reason, alkanes are not actually considered functional groups, since they provide the backbone for everything else.
However, by mixing carbons and hydrogens together, you can get a few other combinations of carbon and hydrogen that are uniquely reactive. This is how we get alkenes (C=C double bonds) and alkynes (C≡C triple bonds).

Hydrocarbon Functional Groups
Benzene is the name of a 6-membered ring with alternating single and double bonds, however “benzene” is not really a functional group since it is the name of a single molecule. The group is actually called aromatics or arenes. Thus, benzene is part of an aromatic functional group.
Remember: The “-R” symbol is used to represent extra hydrocarbon side chains on the molecule that we don’t particularly care about at that moment.
When you extend benzene off of a carbon chain (-R), you get the side chain functional groups phenyl (directly attached to the main chain) and benzyl (extra –CH2 between the benzene and the main chain).
Now that we are done with hydrocarbons, let’s talk about carbonyls.
Intermission: What is a carbonyl and why does it matter?
Carbonyls are just C=O bonds on your molecule. They are NOT true functional groups since they are an essential component of many different functional groups. Some textbooks call them types of “moieties” (a bonding pattern) but I’m just going to call them carbonyls and expect you to remember that they are not functional groups.

Carbonyl is not Technically a Functional Group
2. Functional Groups WITHOUT Carbonyls
When you add single bonded heteroatoms (non-carbon atoms) to alkane chains, you end up getting a bunch of the most essential functional groups for organic chemistry.
You’ll see soon why the “no carbonyls” part is important in a sec. Here’s the list:

List of Functional Groups Without Carbonyls
- When you add any halogen “-X” (Group 7a: -F, -Cl, -Br, -I) you get an alkyl halide (RX)
- Also known as a haloalkane
- Specifically called alkyl fluoride, alkyl chloride, alkyl bromide, alkyl iodide
- When you add any –N (with hydrogens OR carbons attached) you get an amine
- When you add an –OH you get an alcohol (ROH)
- When you add an –OR you get an ether (ROR)
- Memory Trick: “I’m an ether, ROR! (like you are a scary dinosaur)”
- When you add an –SH you get a thiol (RSH)
- When you add a -C≡N you get a nitrile (RCN)
3. Functional Groups WITH Carbonyls
If you take the same functional groups we just memorized and place a carbonyl next to them, you get entirely new compounds.

List of Carbonyl Functional Groups
When you add a carbonyl to an alkane you actually get two functional groups:
- When the carbonyl is on the end of carbon chain (terminal) it's called an aldehyde
- When the carbonyl is in the middle of the carbon chain (internal) it's called a ketone
When you place carbonyls next to the heteroatom-containing functional groups we learned in point 2, many of the new functional groups are given similar names, so it's easy to remember which functional groups pair up with which.
- Alkyl Halide + Carbonyl = Acid Halide aka Acyl Halide (COX)
- Acyl chloride, acyl bromide, acyl iodide (acyl fluoride is rarely observed)
- Amine + Carbonyl = Amide (CONH3, CONRH2, CONR2H, CONR3)
- Alcohol + Carbonyl = Carboxylic Acid (COOH or CO2H)
- Ether + Carbonyl = Ester (COOR or CO2R)
4. Putting It All Together
The way I’ve organized this chart, similar groups are aligned horizontally and similar pairs are lined up vertically. Now it's time for you to put this into practice.
Can you name the 17 functional groups and fill in the blank spaces in the functional group table without looking above?
 Blank list of functional groups
Blank list of functional groups
Now, what’s the difference between a primary alcohol and a tertiary alcohol? We’re going to talk about how to name the degrees of each functional group.
PART 2: DETERMINING FUNCTIONAL GROUP DEGREE
The best way to introduce the concept of degrees is actually to go back to the example of hydrocarbons.

Example of Hydrocarbon Degrees
Both carbons and hydrogens can be named as methyl (0°), primary (1°), secondary (2°), tertiary (3°), etc.
The highlighted carbon and hydrogen in this example are both secondary (2), but for very different reasons.
- The carbon is secondary because it is directly attached to two other carbons
- The hydrogen is also secondary because it is connected to a carbon that is connected to two other carbons (indirectly attached)
See the difference? Hydrogens get their degree from the carbon they are attached to.

This rule can be generalized to other atoms and functional groups as well.
- Atoms that can form multiple bonds (i.e. C, N) derive degree from direct attachment
- Atoms/groups that can only form one bond (i.e. –H, –X, –OH) derive degree from indirect attachment
Not all functional groups get degrees by the way. In my functional groups video module, I call the functional groups that get degrees the “A Team”, because they all start with the letter A.
- Alcohol (R-OH)
- Alkyl Halide (R-X)
- Amine (NH3, NRH2, NR2H, NR3)
- Amide (CONH3, CONRH2, CONR2H, CONR3)
Just by observing these condensed formulas, can you predict which of these groups will derive degree from direct attachment (like carbon) because they can form multiple combinations of bonds?

Functional Group Examples
Here’s a video of Jonathan identifying functional groups in the gigantic anti-rejection molecule called everolimus (zortress). Can you find them all (including degrees)?

Functional Group Answers:
2. Aldehyde. It is a carbonyl at the end of a chain with no heteroatoms next to it.
- Even though it starts with “A” sadly aldehyde is not on the A team!
3. Secondary (2°) amine because the nitrogen is directly attached to two carbons
4. Ester. Looks more confusing in condensed formula, but you need to be able to recognize this.
5. Primary (1°) alkyl halide (chloride) because the chlorine is indirectly attached to 1 carbon.
- (Aka the carbon that the chlorine is attached to is only attached to 1 carbon)
6. Tertiary (3°) amide because the nitrogen is directly attached to 3 carbons
7. Methyl (0°) alcohol because the oxygen is indirectly attached to zero carbons
- (Aka the carbon that the oxygen is attached is not connected to any other carbons)
8. Ether. I’m an ether, R-O-R!
9. Ketone. This is a carbonyl in the middle of a chain with no other heteroatoms next to it. Easy to recognize that this is a ketone.















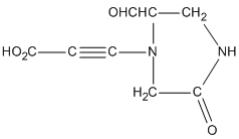





 Blank list of functional groups
Blank list of functional groups





