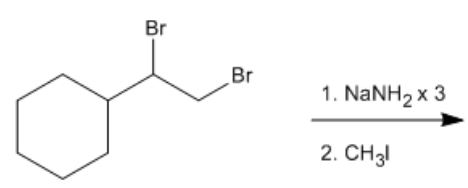
Triple bonds, specifically in hydrocarbons known as alkynes, can be transformed into strong nucleophiles through a unique property of terminal alkynes. Terminal alkynes possess a hydrogen atom at one end and exhibit unusual acidity due to their hybridization. This acidity can be understood through the concept of hybridization effects, which influence the acidity of compounds. In this context, terminal alkynes have sp hybridization, meaning they have 50% s character. This high s character contributes to their acidity, with a pKa value of 25, which is relatively low for hydrocarbons.
To utilize this acidity, a strong base is required to deprotonate the terminal alkyne, resulting in the formation of a strong nucleophile known as a sodium alkynide. Common strong bases for this reaction include sodium amide (NaNH2) or sodium hydride (NaH). When a strong base is introduced, it can effectively remove the acidic hydrogen from the terminal alkyne, leading to the generation of a negatively charged species at the carbon atom involved in the triple bond.
The reaction can be illustrated as follows: when sodium hydride dissociates, it produces Na+ and H-. The H- ion acts as the base, reacting with the hydrogen of the terminal alkyne. This process results in the formation of a sodium alkynide, characterized by a negative charge on the carbon of the triple bond. This transformation allows the previously stable hydrocarbon to act as a potent nucleophile, opening up new pathways for chemical reactions.