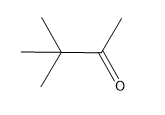
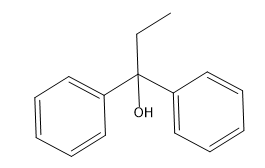
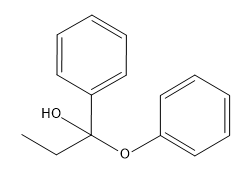
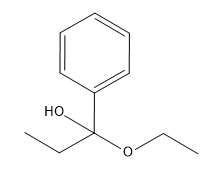
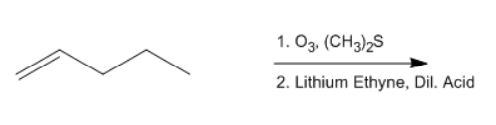
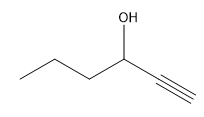
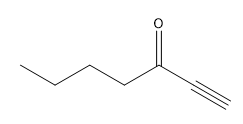
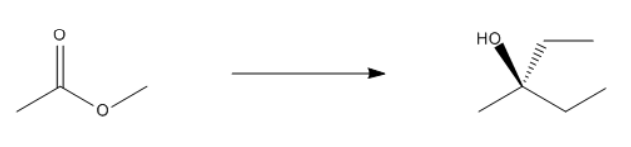
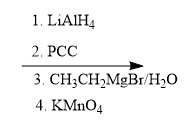
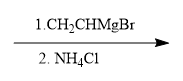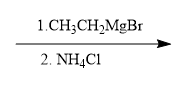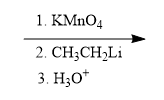In organometallic chemistry, predicting the products of reactions involves understanding the reactivity of organometallic compounds, which typically contain a carbon-metal bond. These compounds can undergo various types of reactions, including nucleophilic additions, coupling reactions, and electrophilic substitutions. A key aspect of these reactions is recognizing the role of the organometallic reagent, which often acts as a nucleophile due to the polarized carbon-metal bond.
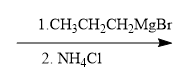
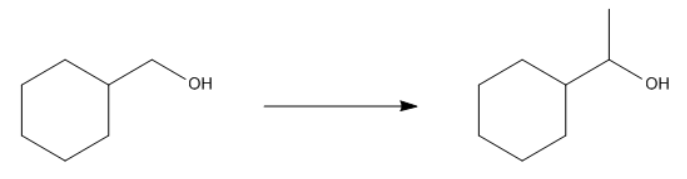
For example, in a nucleophilic addition reaction, an organometallic reagent such as a Grignard reagent (RMgX) can react with carbonyl compounds. The carbon atom in the organometallic reagent attacks the electrophilic carbon in the carbonyl, leading to the formation of an alcohol after subsequent protonation. The general reaction can be represented as:
R-MgX + R'CHO → R-CH(OH)-R' + MgX(OH)
In multi-step reactions, it is essential to identify each step and the intermediates formed. For instance, if an organometallic compound reacts with an alkyl halide, it may first form a new organometallic compound before undergoing further reactions. This requires a solid understanding of reaction mechanisms and the stability of intermediates.
As you work through the practice problems, remember to consider the functional groups present, the nature of the organometallic reagent, and the potential for rearrangements or side reactions. By applying these principles, you can systematically predict the products of complex reactions involving organometallics.