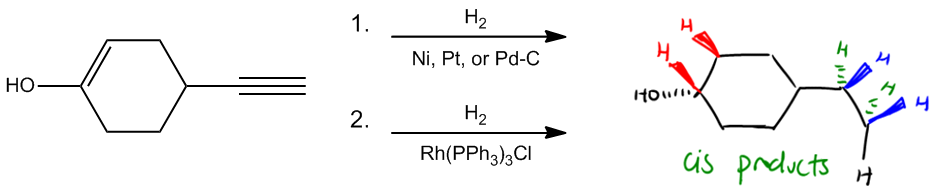
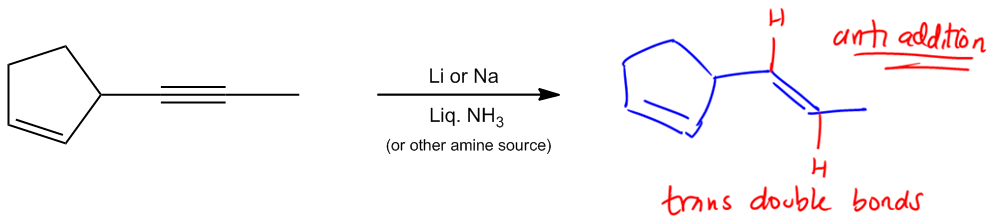
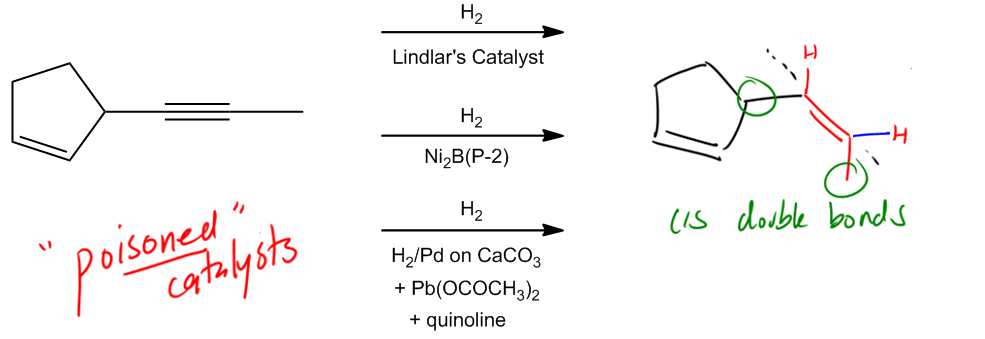
Hydrogenation is a chemical reaction that involves the addition of hydrogen (H2) to unsaturated compounds, specifically those containing double or triple bonds. The simplest form of hydrogenation is known as full saturation, where these unsaturated bonds are completely converted into single bonds, resulting in alkanes. This process can be achieved using two primary methods: catalytic hydrogenation and Wilkinson's catalyst.
In catalytic hydrogenation, hydrogen gas is introduced over a catalyst, typically platinum, palladium, or nickel. This method effectively adds hydrogen across double or triple bonds, transforming them into single bonds. Alternatively, Wilkinson's catalyst, which consists of rhodium combined with triphenylphosphines and chlorine, serves a similar purpose. Although the reagents for Wilkinson's catalyst may appear complex, their function remains straightforward: to saturate unsaturated hydrocarbons.
It is important to note that while full saturation eliminates double and triple bonds, it does not affect cyclic compounds (rings). Rings inherently lack two hydrogen atoms due to the connection of their ends, and thus remain unchanged during the hydrogenation process.
Another key aspect of hydrogenation is the stereochemistry involved, specifically syn addition. This term indicates that the hydrogen atoms are added to the same side of the double or triple bond, resulting in cis products. For example, when hydrogen is added to a double bond, both hydrogen atoms can originate from the same side, influencing the orientation of any substituents attached to the carbon atoms involved in the bond.
When performing full saturation, the resulting structure retains the same sigma framework, meaning that all sigma bonds remain intact while pi bonds are eliminated. For a triple bond, four hydrogen atoms are added—two from the front and two from the back—while ensuring that any existing hydrogen atoms on the carbon atoms are accounted for. This comprehensive addition results in a fully saturated alkane, devoid of any pi bonds.
In summary, full saturation through catalytic hydrogenation or Wilkinson's catalyst effectively removes all double and triple bonds, converting unsaturated hydrocarbons into saturated alkanes while preserving the integrity of cyclic structures and adhering to specific stereochemical outcomes.