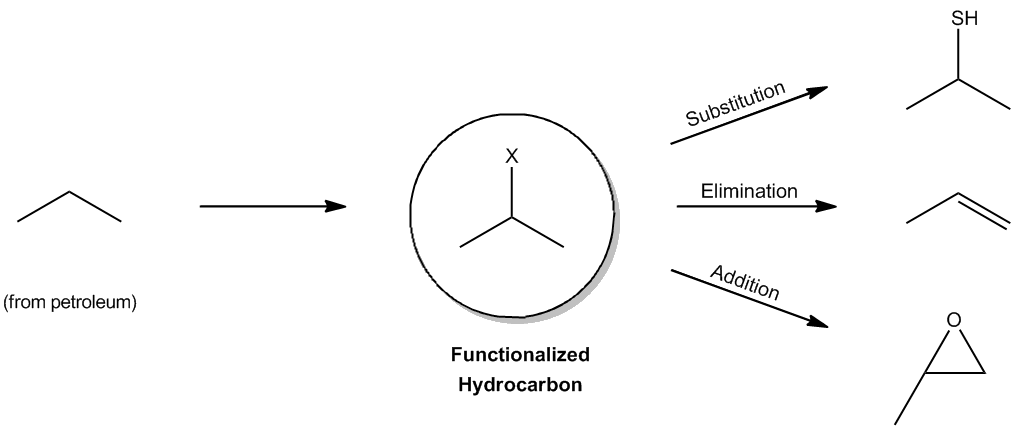
Alkanes, while abundant and stable, are not classified as functional groups due to their limited reactivity. These hydrocarbons, primarily derived from petroleum, are characterized by their resistance to chemical reactions, making them seem unremarkable at first glance. However, they can undergo radical reactions, which are initiated by high-energy radicals that can interact with these otherwise unreactive molecules.
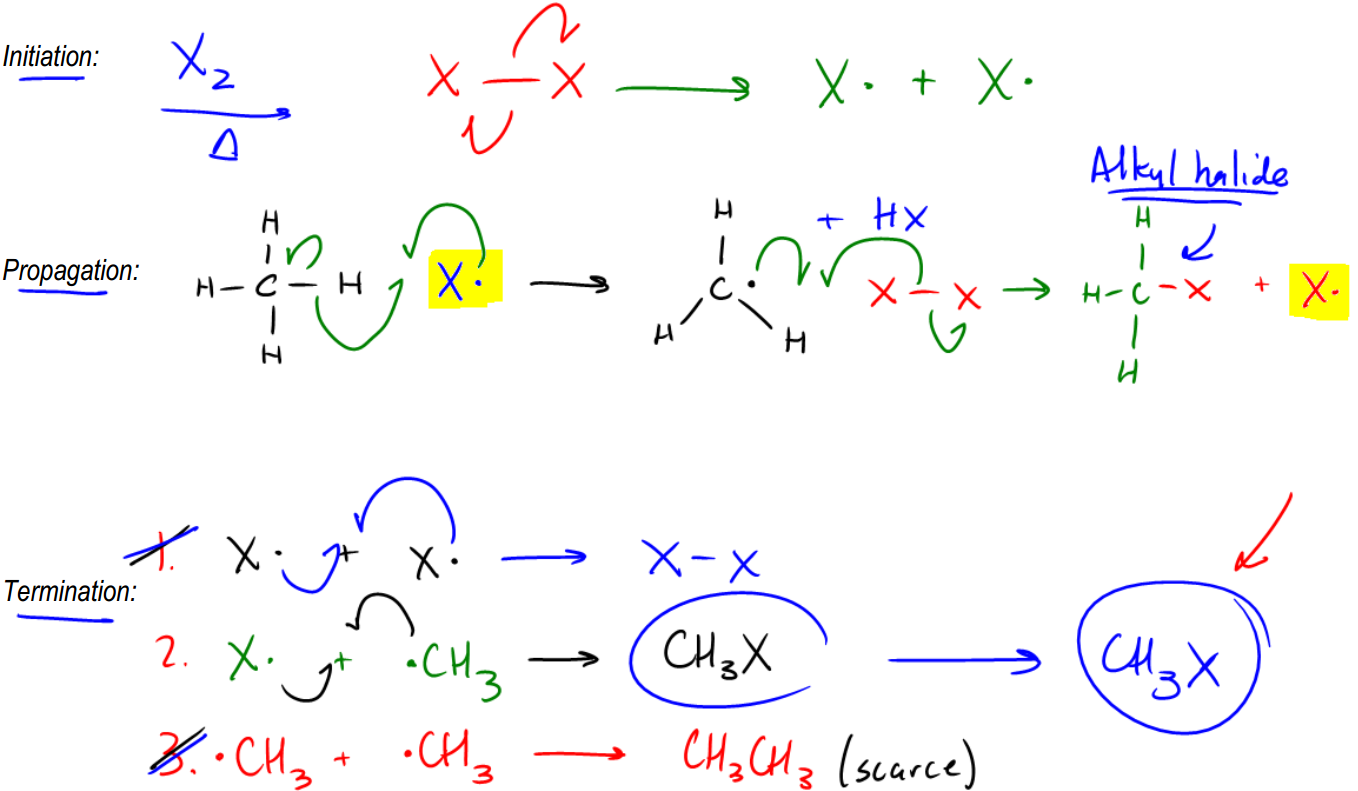
In the presence of radicals, alkanes can react with halogens, leading to the formation of alkyl halides. This transformation is significant because it introduces a functional group into the alkane, effectively converting it into a functionalized hydrocarbon. The presence of the alkyl halide opens the door to a variety of organic reactions, including substitution, elimination, and addition reactions. This process marks a crucial step in organic synthesis, as it allows for further chemical modifications and the creation of more complex organic compounds.
Understanding the mechanism of radical reactions with alkanes is essential for grasping the foundational concepts of organic chemistry. The ability to manipulate these stable hydrocarbons through radical halogenation is a key technique that enables chemists to synthesize a wide range of organic molecules.