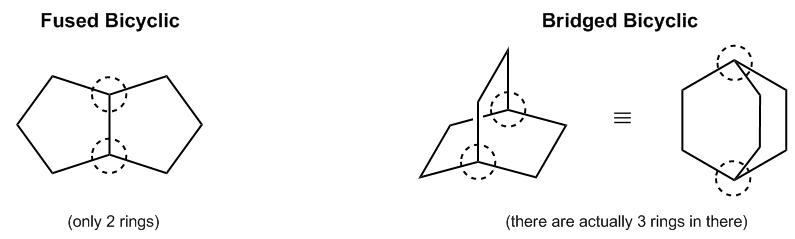
Bicyclic compounds are categorized into two main types: normal bicyclics and bridged bicyclics. Normal bicyclics consist of a single structure with two rings connected by one bond. In this case, you can identify the two rings as ring number one and ring number two, forming a straightforward bicyclic structure.
On the other hand, bridged bicyclics are more complex, containing a total of three rings. Visualizing this can be challenging, but you can think of it as having a base ring at the bottom, with two additional rings extending from either side. These three rings are interconnected through specific atoms known as bridgehead atoms, which serve as the connection points between the rings.
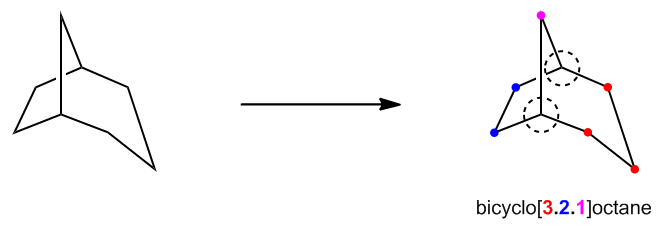
Interestingly, both the three-dimensional (3D) and two-dimensional (2D) representations of these bridged bicyclics depict the same molecular structure. For instance, the base ring can often be represented as a cyclohexane, while the bridge connecting the rings can be illustrated in a different color to distinguish it. This dual representation highlights the importance of understanding both planar and spatial configurations of bicyclic compounds.
When it comes to naming bicyclic compounds, the nomenclature differs significantly from that of monocyclic alkanes due to their increased complexity. This complexity necessitates a unique approach to naming, which reflects the structural intricacies of these compounds.