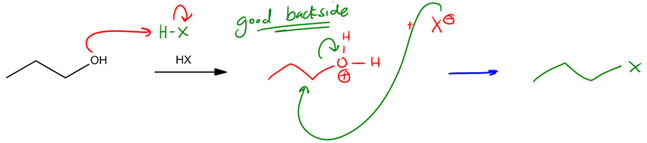
Alcohols are a significant functional group in organic chemistry, but they present a challenge due to their poor leaving group ability. A good leaving group is characterized by its stability once it departs from the molecule. When an alcohol leaves, it forms a hydroxide ion (OH-), which is a strong base and thus unstable. This instability makes alcohols less favorable in reactions that require effective leaving groups.
Fortunately, there are strategies to enhance the leaving group capability of alcohols. One effective method is to convert the alcohol into an alkyl halide. Alkyl halides, represented by the formula R-X (where X can be iodine, bromine, or chlorine), are excellent leaving groups because the halide ion (X-) is stable and can easily depart from the molecule. The electronegativity of halogens allows them to accommodate a negative charge without destabilization.
Another viable pathway involves the formation of sulfonate esters, which are also known for their excellent leaving group properties. This process entails converting an alcohol into a sulfonate ester, thereby enhancing its ability to act as a leaving group in various reactions. Understanding these transformations is crucial for effectively utilizing alcohols in organic synthesis.