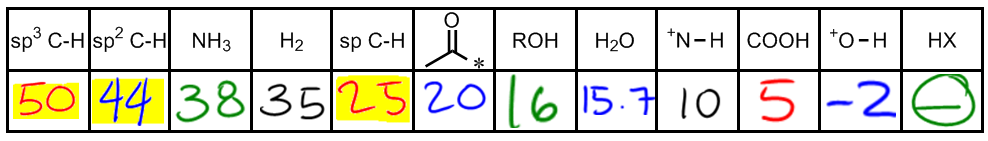
Understanding the concept of pKa is essential in organic chemistry, as it helps determine the acidity of various molecules. pKa values indicate how well a substance can donate protons (H+ ions), with lower pKa values signifying stronger acids. While memorization of specific pKa values is often necessary, grasping the underlying principles can enhance comprehension.
Starting with the least acidic compounds, alkanes, which are sp3 hybridized, have a pKa around 50. This high value reflects their lack of reactivity, as they do not possess dipoles, charges, or double bonds that would facilitate proton donation. Next, alkenes (sp2 hybridized) exhibit slightly better acidity with a pKa of 44 due to the presence of double bonds, which increase reactivity.
Amines, characterized by an H atom bonded to nitrogen, have a pKa of 38, indicating they are still relatively weak acids. Diatomic hydrogen (H2) has a pKa of 35, which is another value to memorize. Alkynes, with sp hybridization, show improved acidity with a pKa of 25, as the triple bond enhances their ability to donate protons.
Alpha hydrogens, which are attached to carbons adjacent to carbonyl groups, have a notably lower pKa of 20. This increased acidity is attributed to tautomerization, a concept that will be explored further in advanced studies. Alcohols and water both have a pKa of approximately 16, serving as a reference point for comparing other acids.
Moving to stronger acids, a positively charged nitrogen with at least one hydrogen has a pKa around 10, indicating significant acidity. Carboxylic acids, which include compounds like acetic acid, have a pKa of about 5. This value is derived from calculations involving their dissociation in solution.
As we approach stronger acids, an oxygen atom with a positive charge and at least one hydrogen has a pKa of around -2, marking it as a very strong acid. Finally, strong acids such as HCl, HBr, and HI are grouped together with pKa values that are negative, indicating their high acidity. While specific negative values may vary, the key takeaway is that these acids are significantly more potent than those with positive pKa values.
In summary, the pKa values of various organic compounds range widely, from alkanes at 50 to strong acids with negative values. Familiarity with these values and the factors influencing acidity will be crucial for success in organic chemistry.