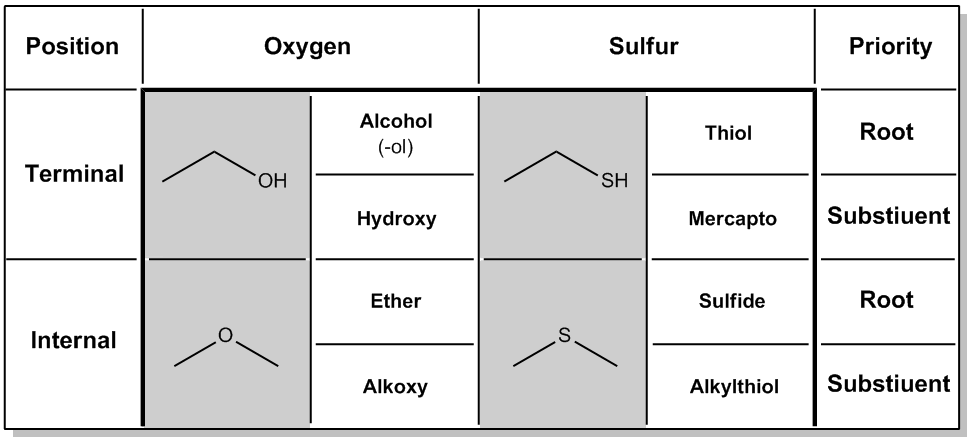
The nomenclature of sulfur-containing compounds closely resembles that of oxygen compounds due to their similar positions in the periodic table. Sulfur, located below oxygen, allows for analogous functional groups, such as alcohols and ethers, to have sulfur counterparts. Understanding these relationships is crucial for correctly naming these compounds.
Two primary functional groups for oxygen are alcohols, characterized by a terminal hydroxyl group (–OH), and ethers, which contain an internal oxygen atom between two carbon chains. When naming compounds, the presence of higher priority functional groups can affect the naming of these oxygen-containing groups. For instance, if an alcohol is present alongside a carboxylic acid, the alcohol is referred to as a hydroxy substituent instead of being the root name.
Similarly, sulfur can form terminal and internal structures. A terminal sulfur group (–SH) is analogous to an alcohol and is named a thiol. If a higher priority group, such as a carboxylic acid, is present, the thiol is named as a mercapto substituent. The term "mercapto" derives from Latin, meaning sulfur, and is used in the same context as hydroxy when naming substituents.
For sulfur ethers, the naming convention is to refer to them as sulfides, which parallels the naming of ethers. In the IUPAC system, sulfides can also be named as alkyl thiols, similar to how ethers are named as alkoxy groups. This systematic approach helps in understanding the nomenclature of sulfur compounds by relating them to the more familiar oxygen compounds.
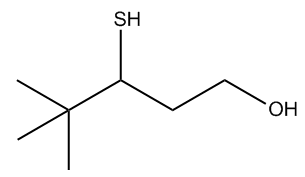
In summary, recognizing the structural similarities between sulfur and oxygen compounds aids in mastering their nomenclature. Remember that alcohols have a higher priority than thiols, which will guide you in determining the root name when both are present in a compound. Practice with specific examples will further solidify your understanding of these naming conventions.