Understanding the mechanisms of organic chemistry is crucial for mastering the subject, as they form the foundation for predicting how molecules interact and react. A key concept in this area is the relationship between stability and reactivity, which are inversely related. This means that as the stability of a molecule increases, its reactivity typically decreases, and vice versa. Recognizing this relationship is essential for predicting the behavior of different compounds.
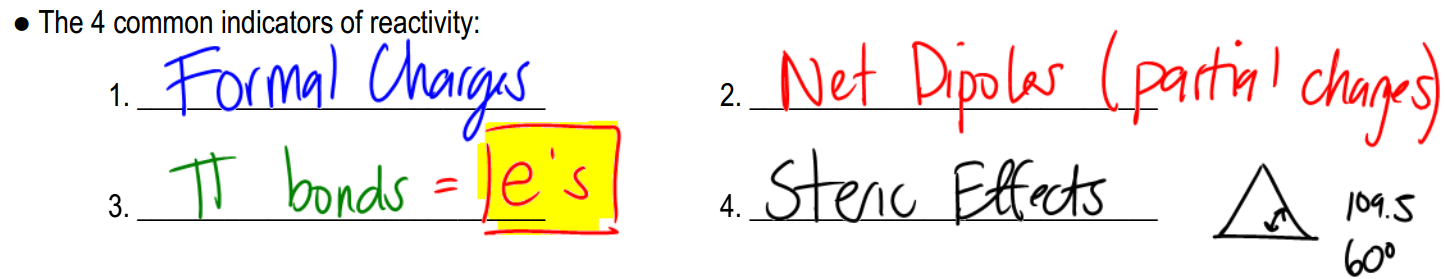
To assess the reactivity of molecules, there are four primary indicators to consider:
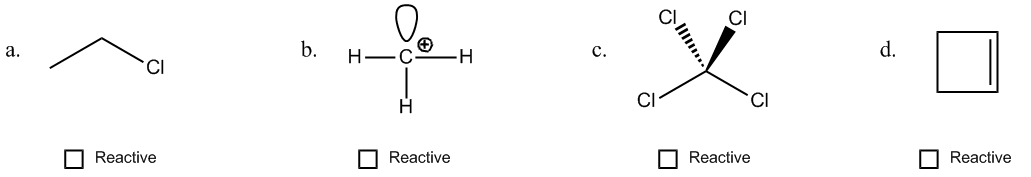
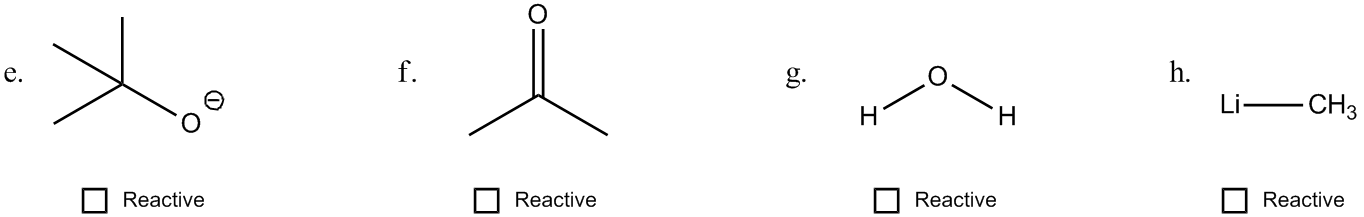
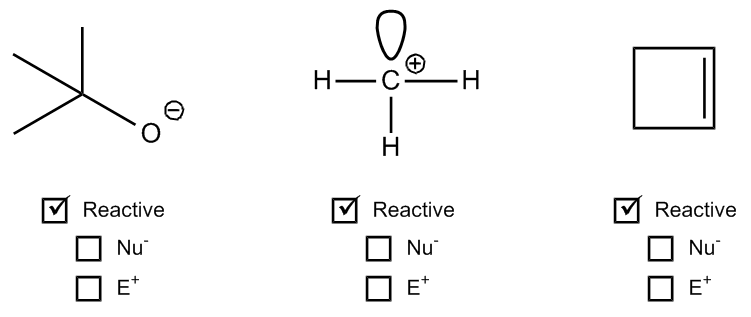
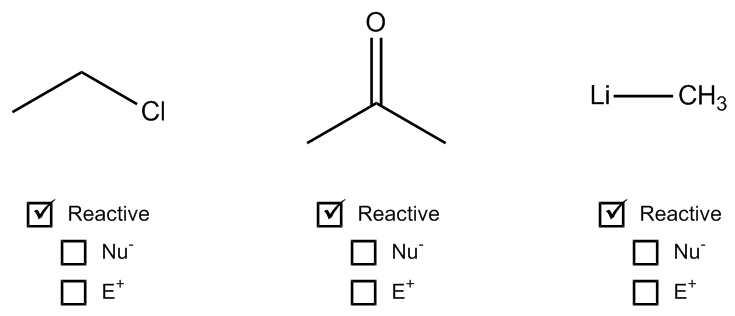
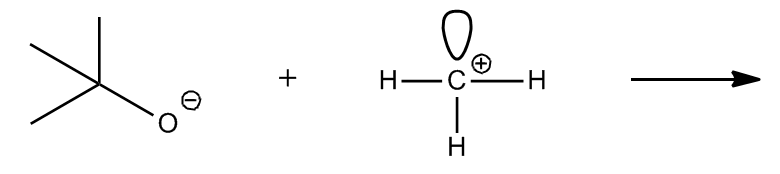
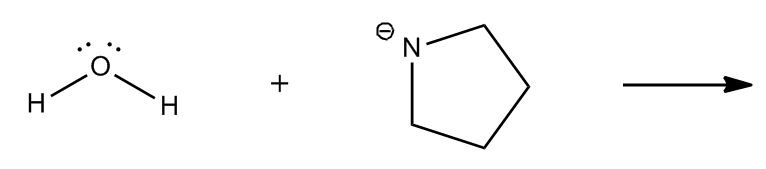
1. Formal Charges: When an atom has a formal charge, it indicates that it is not at its ideal bonding preference, possessing either too many or too few valence electrons. This imbalance drives the atom to seek stability through reactions, making it more reactive.
2. Net Dipoles: A net dipole occurs when there are asymmetrical dipoles that do not cancel out, resulting in partial charges within the molecule. The presence of these partial charges signifies reactivity, as they create regions of positive and negative charge that can interact with other molecules.
3. Pi Bonds: Pi bonds are generally weaker than sigma bonds and serve as a source of electrons that can be easily broken. This characteristic makes molecules with pi bonds more reactive, as the bonds can be disrupted during chemical reactions.
4. Steric Effects: Steric effects arise from the spatial arrangement of atoms within a molecule. For example, ring strain in small cyclic compounds can create instability due to bond angles that deviate from their ideal values. This strain contributes to the reactivity of the molecule.
By evaluating these four factors—formal charges, net dipoles, pi bonds, and steric effects—students can determine the reactivity of various organic compounds. Engaging in exercises that involve analyzing different molecules for these indicators will reinforce understanding and application of these concepts in organic chemistry.


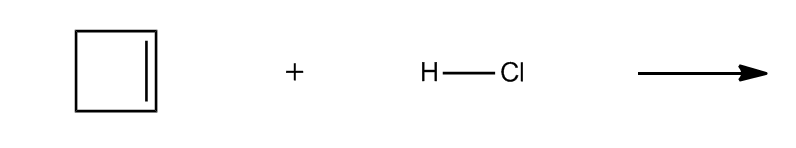


 1 student found this helpful
1 student found this helpful