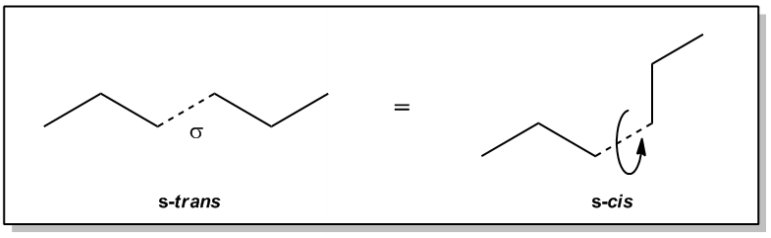
Conformers are essential concepts in organic chemistry, representing the different spatial arrangements of molecules that can occur without any chemical change. This phenomenon primarily arises from the rotation around single bonds, known as sigma bonds. Sigma bonds consist of one region of overlap between atomic orbitals, allowing for free rotation without altering the connectivity of the atoms involved. As a result, when a molecule rotates around a sigma bond, it does not form an isomer; instead, it simply adopts a different conformation.
For instance, consider hexane, a six-carbon chain. The sigma bond in hexane can rotate, leading to various conformations. If we analyze the arrangement of substituents around a bond, we can describe these conformations as either "trans" or "cis" if they were part of a double bond. However, since single bonds can rotate freely, we refer to these arrangements as conformations rather than fixed isomers. When the larger groups attached to the carbon atoms are on opposite sides, we describe this as a trans conformation, while if they are on the same side, it is referred to as a cis conformation.
In the case of hexane, the molecule can exist in multiple conformations, constantly transitioning between them due to the flexibility of the sigma bonds. This dynamic behavior means that hexane does not always maintain a straight zigzag shape; it can adopt more crumpled or varied forms. These different arrangements of the same molecule are collectively known as conformers, highlighting the importance of understanding molecular flexibility in organic chemistry.