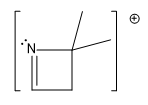
In mass spectrometry, understanding fragmentation patterns is crucial for analyzing molecular structures. The process begins with ionization, where molecules are converted into ions, and the likelihood of this occurring is influenced by a concept known as ionization potential. Ionization potential refers to the energy required to remove an electron from an atom or molecule. Electrons that are held loosely are more easily ionized, while those held tightly require more energy to remove.
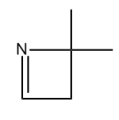
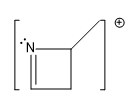
One of the most favorable fragments to ionize is the lone pair of electrons on nitrogen. This is due to nitrogen's reactive nature, making its lone pair highly susceptible to ionization. Following nitrogen, aromatic compounds, such as benzene, exhibit a unique behavior during ionization. The stability of the aromatic ring means that ionization typically occurs at the single bonds attached to the ring rather than disrupting the ring itself. This results in the formation of radical cations without breaking the aromatic structure.
Next in the hierarchy of ionization potential are compounds with double bonds, referred to as vinyl positions. These are slightly more challenging to ionize than aromatics but still easier than other structures. The presence of a double bond provides some stabilization, making it a preferred site for ionization.
Lone pairs on oxygen atoms are also susceptible to ionization, though they have a higher ionization potential compared to nitrogen due to their lower reactivity. Finally, the most difficult fragments to ionize are those attached to single bonds in alkanes. In these cases, there are no stabilizing factors to assist in the ionization process, making it a more energy-intensive task to remove an electron and form a radical cation.
In summary, the order of ionization potential from easiest to hardest is: lone pairs on nitrogen, aromatic compounds, vinyl positions, lone pairs on oxygen, and finally, single bonds in alkanes. Understanding these patterns is essential for predicting the major cations formed during mass spectrometry analysis and can significantly aid in the interpretation of mass spectra.