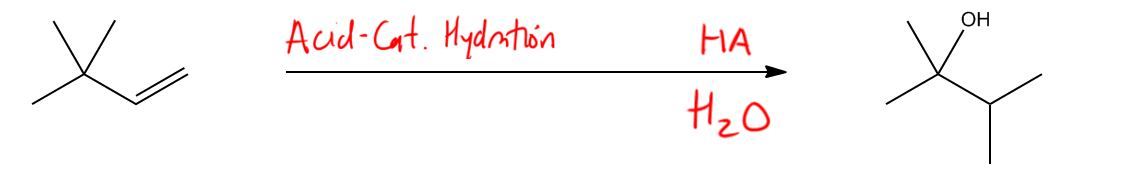
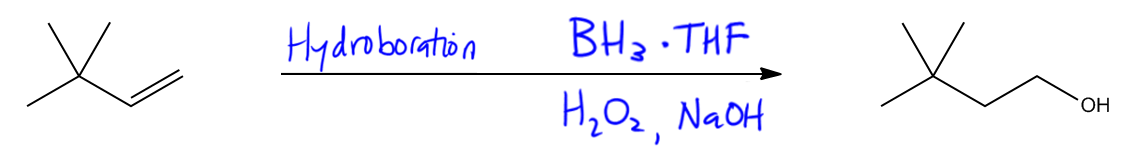Alcohols can be synthesized through various methods, and it's essential to recall the four primary techniques previously discussed. One effective method for introducing a hydroxyl group to a double bond, particularly at a secondary position, is through the reaction known as oxymercuration. This addition reaction is significant because it follows Markovnikov's rule, which states that the more substituted carbon will preferentially bond with the hydroxyl group. The advantage of oxymercuration is that it avoids the formation of carbocations, which can lead to rearrangements during the reaction process.
In the oxymercuration reaction, the reagents used include mercuric acetate, represented as Hg(OAc)2, and water. The water serves as the source of the hydroxyl group that will be added to the double bond. Following this addition, a reduction step is performed to convert the mercurial intermediate into the corresponding alcohol. This reduction is typically achieved using sodium borohydride (NaBH4), which acts as a reducing agent, along with sodium hydroxide (NaOH) to facilitate the reaction.
Overall, oxymercuration is a reliable method for synthesizing alcohols from alkenes, particularly when the goal is to achieve Markovnikov addition without the complications of carbocation rearrangement. Understanding this reaction and its reagents is crucial for mastering alcohol synthesis in organic chemistry.