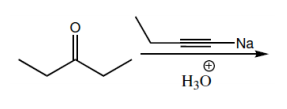
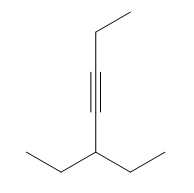
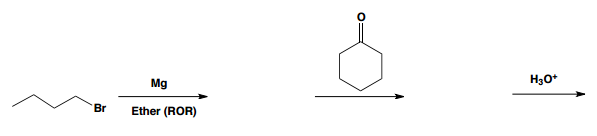
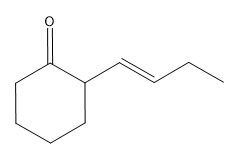
Nucleophilic addition reactions involving organometallic compounds, such as Grignard reagents and organolithiums, are fundamental in organic chemistry. Organometallics are characterized by a metal atom bonded to a carbon atom, which is typically part of an alkyl group. The general formula for a Grignard reagent is represented as R-MgBr, while organolithiums are denoted as R-Li. These compounds are powerful nucleophiles due to the highly ionic nature of the bond between the carbon and the metal, resulting in a significant dipole moment.
In the context of nucleophilic addition to carbonyl compounds like ketones and aldehydes, the nucleophilic carbon (R-) from the organometallic attacks the electrophilic carbon of the carbonyl group. This interaction leads to the formation of a tetrahedral intermediate, which can be represented as R-C(=O)R1R2. The carbonyl oxygen, now bearing a negative charge, is typically followed by a protonation step, resulting in the formation of an alcohol product. The final product can be expressed as R1R2COH, where the new R group from the organometallic is now attached to the carbon that was originally part of the carbonyl.
To summarize, when an organometallic compound reacts with a ketone or aldehyde, the outcome is always an alcohol with a new alkyl group attached to the carbon that was part of the carbonyl. Understanding this mechanism is crucial for effectively utilizing organometallics in synthetic organic chemistry.