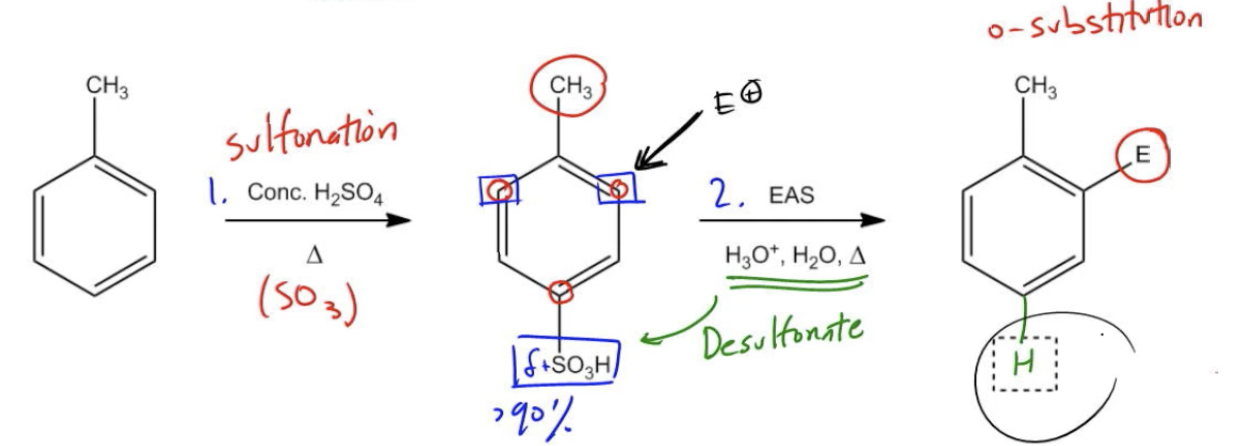
In synthetic organic chemistry, a strategic approach known as blocking is employed to facilitate specific electrophilic aromatic substitution (EAS) reactions, particularly when aiming for ortho substitution on a benzene ring. One of the key reactions in this strategy is sulfonation, which utilizes concentrated sulfuric acid to introduce a sulfonic acid group (–SO₃H) onto the aromatic ring. This reaction is reversible, allowing for the removal of the sulfonic acid group through heating with acid or steam, thus providing a unique synthetic advantage.
When targeting ortho substitution, it is essential to consider the directing effects of substituents already present on the benzene ring. Ortho/para directors typically favor para substitution due to steric hindrance, making it challenging to achieve high yields of ortho products. To overcome this, sulfonation is employed to block the para position, effectively forcing the electrophile to react at the ortho position instead. This is particularly useful when high yields of ortho products (ideally 80% or 90%) are desired.
For instance, when starting with toluene, a sulfonation reaction is performed first, resulting in the formation of a sulfonic acid group predominantly at the para position (over 90% yield). Following this, a second EAS reaction is conducted. The methyl group (–CH₃) acts as an ortho/para director, while the sulfonic acid group serves as a meta director due to its strong electron-withdrawing nature. This combination directs the electrophile to the ortho positions, allowing for successful ortho substitution.
After the electrophilic addition, the final step involves desulfonation using dilute acid, which removes the sulfonic acid group and restores a hydrogen atom at the para position. The result is a product that features the desired ortho substitution, demonstrating the effectiveness of the blocking strategy in synthetic pathways.
In summary, the blocking strategy through sulfonation is a powerful tool in organic synthesis, enabling chemists to selectively achieve ortho substitution by temporarily occupying the para position, thus enhancing the overall yield of the desired product.