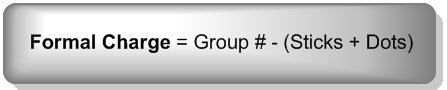Understanding formal charges is essential in the study of chemical bonding, as it directly relates to the bonding preferences of atoms. A formal charge is assigned to an atom when there is a discrepancy between the number of valence electrons an atom desires and the number it actually possesses in a molecule. The desired number of valence electrons corresponds to the atom's group number in the periodic table, while the actual number is determined by counting the bonds (represented as "sticks") and lone pairs (represented as "dots") surrounding the atom.
To calculate the formal charge, you can use a straightforward formula:
\[\text{Formal Charge} = \text{Group Number} - (\text{Number of Bonds} + \text{Number of Lone Pair Electrons})\]
In this equation, the group number indicates how many electrons the atom wants, while the sum of the number of bonds and lone pair electrons gives the actual count of valence electrons. This calculation can often be performed mentally, making it accessible and easy to apply.
It is important to distinguish between formal charge and net charge. The formal charge pertains to individual atoms within a molecule, allowing you to assess whether each atom carries a charge. In contrast, the net charge refers to the total of all formal charges across the entire molecule. This distinction is crucial for understanding molecular stability and reactivity, as the overall charge can influence how a molecule interacts with others.
In summary, formal charges provide insight into the electron distribution within a molecule, helping to predict its behavior and stability. By mastering the calculation of formal charges, you can enhance your understanding of molecular structures and their implications in chemical reactions.