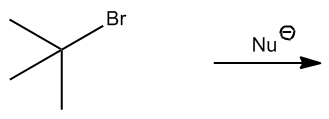The E2 elimination mechanism is characterized by a crucial requirement known as the anticoplanar arrangement. This arrangement is essential for the reaction to proceed, as it allows the orbitals of the leaving group and the beta hydrogen to overlap effectively, facilitating the formation of a new pi bond, which results in the creation of a double bond. Understanding this requirement is fundamental when analyzing E2 reactions.
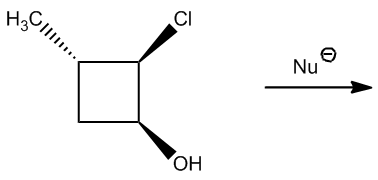
To determine the number of products formed in an E2 reaction, one must first identify the beta hydrogens present in the substrate. Following this, it is necessary to assess which of these beta hydrogens are in the anticoplanar position relative to the leaving group. This two-step process is vital for accurately predicting the outcome of the reaction.
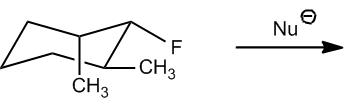
In the context of cyclohexane derivatives, the anticoplanar requirement is referred to as the diaxial requirement. This terminology arises because, in the chair conformation of cyclohexane, the only way for the leaving group and the beta hydrogen to be anti to each other is if they occupy adjacent axial positions. In contrast, equatorial positions do not provide the necessary anti arrangement, as they lead to a gauche interaction instead.
When analyzing E2 reactions involving cyclohexanes, it is important to recognize that the axial positions are less stable than equatorial ones. However, for the elimination to occur, the molecule must adopt a conformation where the leaving group and the beta hydrogen are aligned in an anti configuration. This often requires a conformational change to the less stable axial position, which is critical for the reaction to proceed.
In practice, when evaluating potential E2 reactions, one should start by counting the number of beta hydrogens available. After identifying these hydrogens, the next step is to determine how many of them are in the appropriate anticoplanar or diaxial positions. This analysis will yield the total number of possible products for the E2 reaction, allowing for a comprehensive understanding of the reaction's feasibility.