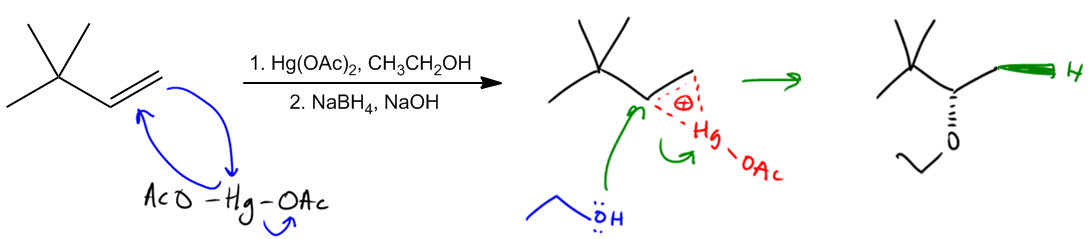
Alkoxymercuration is a synthetic method for producing ethers, closely related to the oxymercuration reaction. The key distinction lies in the choice of nucleophile; instead of using water, alkoxymercuration employs alcohol. This substitution is crucial as it leads to the formation of an ether rather than an alcohol.
The mechanism begins with the double bond of the alkene attacking a mercury species, resulting in the formation of a cyclic ion intermediate. In this step, the mercury atom is bonded to one acetate group (OAc), creating a bridge-like structure. The positive charge on the intermediate indicates that it is a more stable site for nucleophilic attack. In the oxymercuration process, water would typically attack the more substituted carbon (Markovnikov's rule), but in alkoxymercuration, ethanol acts as the nucleophile. The ethanol attacks the positively charged carbon, displacing the mercury and forming the ether.
Following the formation of the ether, a reduction step occurs, typically using sodium borohydride (NaBH4) in the presence of a base. This step does not require a detailed understanding of its mechanism. The reduction converts the mercury to a hydrogen atom and deprotonates the oxygen, resulting in the final ether product, represented as R-O-R, where R denotes the alkyl groups derived from the alcohol.
In summary, alkoxymercuration is a valuable reaction for synthesizing ethers, emphasizing the importance of the nucleophile used in the reaction. Recognizing whether water or alcohol is involved will significantly influence the outcome of the synthesis.