3. Chemical Reactions
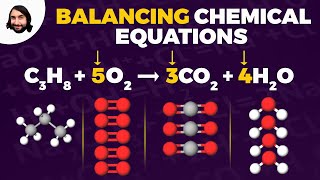
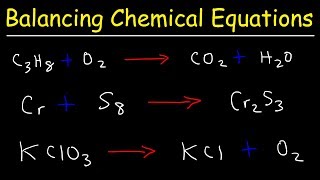
Balancing Chemical Equations
Problem 114
Textbook Question
Textbook QuestionSulfur tetrafluoride 1SF42 reacts slowly with O2 to form sulfur tetrafluoride monoxide 1OSF42 according to the following unbalanced reaction: SF41g2 + O21g2¡OSF41g2 The O atom and the four F atoms in OSF4 are bonded to a central S atom. (a) Balance the equation.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
59sPlay a video:
792
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos