3. Chemical Reactions
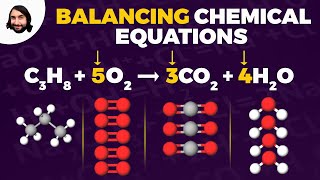
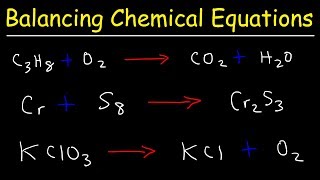
Balancing Chemical Equations
Problem 57a
Textbook Question
Textbook QuestionA 1.50-g sample of quinone 1C6H4O22 is burned in a bomb calorimeter whose total heat capacity is 8.500 kJ>°C. The temperature of the calorimeter increases from 25.00 to 29.49°C. (a) Write a balanced chemical equation for the bomb calorimeter reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
2mPlay a video:
333
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos