3. Chemical Reactions
Balancing Chemical Equations
Problem 146
Textbook Question
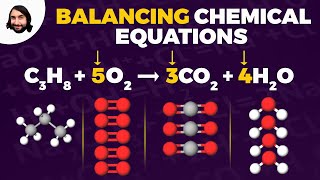
Textbook QuestionWhen a gaseous compound X containing only C, H, and O is burned in O2, 1 volume of the unknown gas reacts with 3 volumes of O2 to give 2 volumes of CO2 and 3 volumes of gaseous H2O. Assume all volumes are measured at the same temperature and pressure. (a) Calculate a formula for the unknown gas, and write a balanced equation for the combustion reaction.
 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
3mPlay a video:
745
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 9 videos