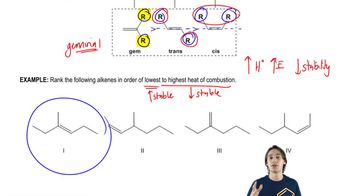
9. Alkenes and Alkynes
Alkene Stability
Problem 7d
Textbook Question
Textbook QuestionA double bond in a six-membered ring is usually more stable in an endocyclic position than in an exocyclic position. Hydrogenation data on two pairs of compounds follow. One pair suggests that the energy difference between endocyclic and exocyclic double bonds is about 9 kJ/mol. The other pair suggests an energy difference of about 5 kJ/mol. Which number do you trust as being more representative of the actual energy difference? Explain your answer. Endoclyclic


 Verified Solution
Verified SolutionThis video solution was recommended by our tutors as helpful for the problem above
Video duration:
4mPlay a video:
1352
views
Was this helpful?
Related Videos
Related Practice
Showing 1 of 2 videos